Introduction
C-reactive protein (CRP) is an acute phase reactant present in healthy individuals in low concentrations. Pathologic conditions like bacterial infections, inflammations or tissue destruction are followed by CRP concentration increase due to the release of proinflammatory cytokines (1). Bacterial endotoxins are the strongest stimulators of inflammatory acute phase reaction (2). The highest CRP concentrations are caused by infections by Gram-negative bacteria (3).
The measurement of C-reactive protein concentration is of extraordinary importance in the diagnosis and follow-up of infection in preterm infants and newborns (1). The onset of sepsis in the newborns may manifest by unspecific symptoms and is diagnosed only on the basis of laboratory findings. Untimely diagnosis of bacterial sepsis is one of the major causes of morbidity and mortality in preterm infants and newborns (2,3,4,6,7).
There is a considerable number of publications about causes, difficulties in making timely diagnosis, diagnostic significance of certain mediators of inflammation in sepsis and treatment, of neonatal sepsis (criteria for instituting and discontinuing therapy (2–6, 8–12).
Recommendations of some institutions are that CRP, though unspecific, has the best diagnostic value as an individual test, is an indicator in therapy onset and monitoring, and may also be used as an indicator to identify the time-point when antibiotic therapy may be safely discontinued (1,2,6,10,11).
Clinical significance of CRP concentration increase within the reference interval induced many manufacturers to develop tests for CRP determination particularly designed to measure very low concentrations. Manufacturers of both nephelometric and turbidimetric reagents turned to latex micro-polymers to achieve the necessary sensitivity so that laboratories have at their disposal several reagent types for CRP determination: highly linear for samples analyzed for general inflammation and infection, and sensitive (SCRP) and highly sensitive (HSCRP) that are employed in neonatology and assessment of the risk of cardio- and cerebrovascular diseases.
The Randox company produced a new combined Full Range CRP assay, characterized by the measuring range of 0.1-160.0 mg/L. When applying Olympus reagents for the same measuring range, we use two reagents and three applications. Considering the importance of early detection of sepsis in preterm infants and newborns, we examined the new reagent on samples from this population. In comparison to the previously applied analytic procedure, expected advantage of the examined reagent was that the use of one reagent instead of a combination of two reagents and three applications should contribute to faster diagnosis of sepsis in such a sensitive population and also to reduced measurement costs.
Aim
Analytical assessment of a new combined Randox Full Range assay for determination of CRP concentration in comparison to Olympus reagents as reference reagents.
Materials and methods
Serum CRP concentration was determined by the combined Full Range CRP assay produced by the Randox company (CP3847) and compared with two reagents produced by the Olympus company (OSR6147 and OSR6185) that are routinely used in the Clinical Institute of Chemistry, Sestre milosrdnice Clinical Hospital.
The Randox Full Range assay uses latex micropolymer-enhanced immunoturbidimetric test which allows determination of CRP concentration in the serum/plasma in the 0.1-160.0 mg/L range. The reagent was applied on the Olympus AU 640 using the application recommended by the Randox company. The original calibrator by the Randox company (CP2499) was used, as well as High Sensitivity CRP Control 2 (CP2477) and CRP Control samples.
The Olympus reagents used for comparison to the Randox Full Range CRP assay were:
1) Olympus System reagent for CRP (OSR 6147), an immunoturbidimetric reagent for the 5.0-300.0 mg/L concentration range.
The original calibrator by the Olympus company was employed for calibration (ODR3021 Serum Protein Multi-Calibrator), and original control samples by the Olympus company for quality control: ITA Control Sera Level 1 (ODC0014), ITA Control Sera Level 2 (ODC0015) and ITA Control Sera level 3 (ODC0016).
2) Olympus CRP Latex reagent (OSR6185), an immunoturbidimetric reagent intended for early diagnosis of infection in preterm infants and newborns. Two applications are available for this reagent:
sensitive - for the 0.5-20.0 mg/L concentration range (SCRP)
highly sensitive - for the 0.05-2.00 mg/L concentration range (HSCRP).
Original calibrators by the Olympus company were used for calibration (ODR3031 – contains five calibration solutions, calibration solutions 3, 4 and 5 are used for sensitive application, while calibration solutions 1 and 2 for highly sensitive application). For quality control, Olympus CRP (Latex) Control Sera (ODC0013 - Control 1 and Control 2) were used.
Analytical evaluation included within-run imprecision, between-run imprecision, inaccuracy and comparative determinations of CRP concentration in the samples of newborns and preterm infants. This study did not require collection of additional blood samples; it involved the use of routine analytical samples.
Imprecision
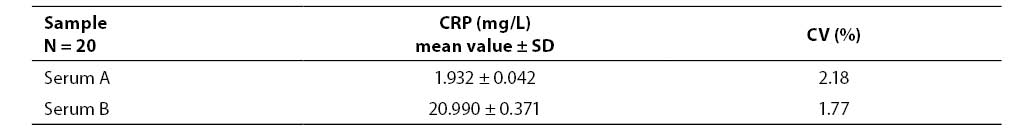
Within-run imprecision was determined by multiple (20 x) measurements of serum sample A and serum sample B and expressed as a coefficient of variation (CV %).
Between-run imprecision was determined by measuring serum sample C in triplicate during 10 days and expressed as a coefficient of variation (CV %).
Inaccuracy
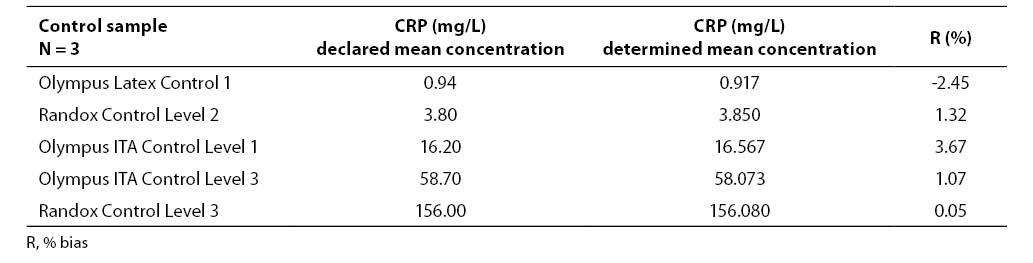
Examination of inaccuracy was performed by measuring CRP concentration in the samples of five different commercial control sera in triplicate: Olympus Latex Control 1 (ODC 0013), Olympus ITA Control Sera Level 1 (ODC0014), Olympus ITA Control Sera Level 2 (ODC0015), Olympus ITA Control Sera level 3 (ODC0016), and Randox Control Level 3 (CP2481). Inaccuracy was expressed as a percentage of bias (R%) of the determined mean value from the declared mean value.
Comparative measurements
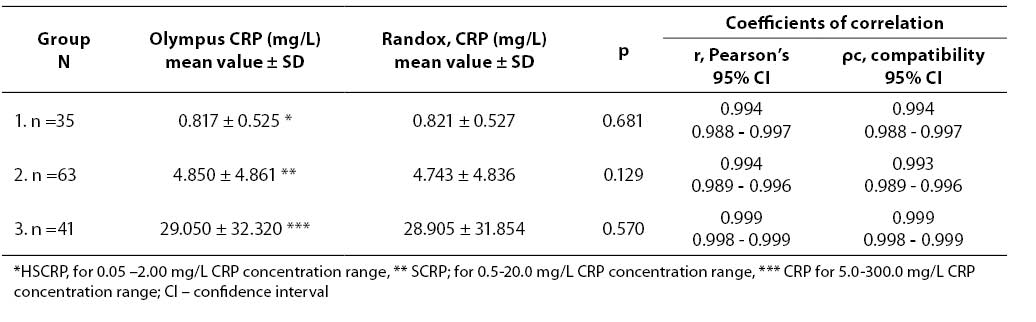
A total of 139 samples from preterm infants and newborns were divided in three groups: group 1, n=35, with CRP concentration lower than 2.00 mg/L; group 2, n=63, CRP concentration between 2,0–20,0 mg/L; group 3, n=41, CRP concentration in the 5.0-300.0 mg/L range. Group 1 was analyzed using Olympus Latex reagent (OSR6185), a highly sensitive application (HSCRP, 0.05-2.00 mg/L measuring range), and the Randox Full Range CRP assay (CP 3847); group 2 was examined using Olympus Latex reagent (OSR 6185), a sensitive application (SCRP, 0.5-20.0 mg/L measuring range) and the Randox Full Range CRP assay (CP 3847); and group 3 using Olympus CRP reagent (OSR 6147, 5.0-300.0 mg/L measuring range) and the Randox Full Range CRP assay (CP 3847).
Statistical processing of results
Results were processed using the statistical program MedCalc (Ver. 9.0.1.0.; Franck Schoonjans, Belgium). Statistical significance of differences was examined by Student T-test. Coefficients of correlation (Pearson’s and coefficient of concordance) were calculated, and linear regression analysis performed according to Passing-Bablok. A change was considered statistically significant if p<0.05.
Results
Results of examining imprecision and inaccuracy (mean value, standard deviation, coefficient of variation, % of bias) are presented in Tables 1-3.
Table 1. Results of within-run imprecision analysis
Table 2. Results of between-run imprecision analysis
Table 3. Results of imprecision analysis
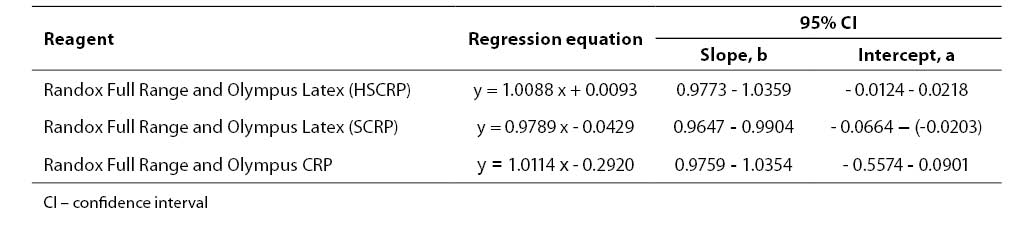
Results of the statistical processing of results of comparative determinations of CRP levels in patient samples (mean value, standard deviation, statistical significance of differences, coefficients of correlation, linear regression analysis according to Passing-Bablok) are presented in Tables 4-5.
Tablica 4. Statistical significance of differences and coefficients of correlation obtained by comparative determination of CRP concentration in three subject groups using reagents by two different manufacturers
Tablica 5. Regression analysis according to Passing-Bablok
Schematic presentation of linear regression analysis according to Passing-Bablok is laid down in Figures 1-3.
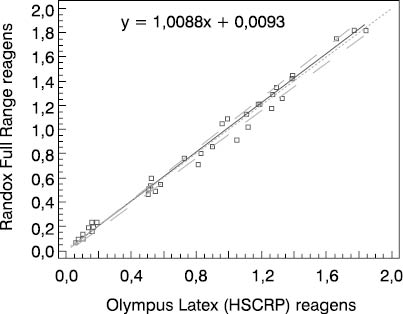
Figure 1. Schematic presentation of comparative determinations of CRP concentration by using the Randox Full Range assay and Olympus Latex (HSCRP) reagent in group 1
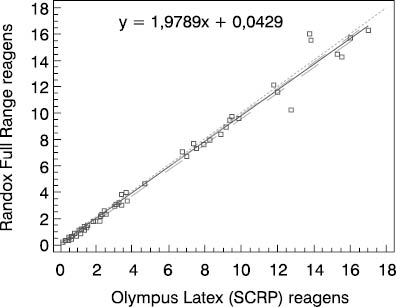
Figure 2. Schematic presentation of comparative determinations of CRP concentration by using the Randox Full Range assay and Olympus Latex (SCRP) reagent in group 2
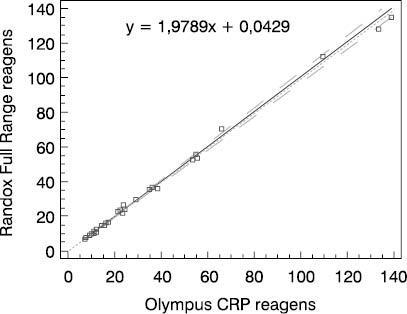
Figure 3. Schematic presentation of comparative determinations of CRP concentration by using the Randox Full Range assay and Olympus CRP reagent in group 3
Discussion
The results obtained by examining the Randox Full Range assay for measuring CRP concentration demonstrated satisfactory imprecision, both within-run- (CV ≤ 2.18%) and between-run imprecision (CV ≤ 1.64%). Satisfactory results were also achieved during examination of inaccuracy (R ≤ 3.67%).
We compared two reagents by different manufacturers in three subject groups (three different concentration ranges). Comparative results of linear regression analysis according to Passing-Bablok showed very good agreement of the two methods as the slope was close to 1.0 and almost equal in all three groups, while the intercept was low. In group 2 which involved 0.5-20.0 mg/L CRP concentration as the measuring range, the results obtained by the Randox Full Range assay differed by a slight constant (within confidence interval for intercept, 0 not included, a= -0.0429). The coefficients of correlation calculated for each subject group showed a high degree of correlation between the Randox Full Range assay and Olympus reagents. Besides, the coefficient of concordance did not differ from the Pearson’s coefficient, allowing us to conclude on the actual, almost ideal correlation between CRP concentrations obtained by using the foregoing two reagents. The coefficient of concordance (ρc) is a measure of precision and accuracy. It comprises the Pearson’s coefficient of correlation (r) and a correction factor (Cb) which is a measure of the bias from the best correlation curve at a slope of 450 (ρc= r x Cb).
Therefore, we may conclude that the Randox Full Range assay could replace Olympus reagents which were used as reference reagents in this study since their analytical reliability has been confirmed by long-term use.
The results obtained are in consistence with results by other authors who compared the same reagents (within-run imprecision: CV ≤ 3.3%; between-run imprecision: CV ≤ 3.7%; results of regression analysis of comparative determinations of CRP concentration in patient samples: r2 ≥ 0.9956) (13).
Our results showed that determination of CRP concentration was equally reliable and reproducible with both reagents. The advantage of the Randox Full Range CRP assay is that a single reagent covers the concentration range from 0.1 to 160.0 mg/L, the fact that is of particular importance for pediatric samples as it allows faster availability of results, whereas the Olympus company employs two reagents and three applications for the same range. The advantage of the Olympus CRP reagent is its very broad measuring range, i.e. 5.0-300.0 mg/L which is very suitable for patients from other departments and wards (department of surgery and intensive care units).
References
1. Jaswal RS, Kaushal RK, Goel A, Pathania K. Role of C-reactive protein in deciding duration of antibiotic therapy in neonatal septicemia. Indian Pediatr 2003;40:880-3.
2. Poschl JM, Hellstern G, Dertlioglou N, Ruef P, Meyburg J, Beedgen B, et al. Six day antimicrobial therapy for early-onset group B streptococcal infection in near-term and term neonates. Scand J Infect Dis 2003;35:302-5.
3. Heiskanen-Kosma T, Korppi M. Serum C-reactive protein cannot differentiate bacterial and viral aetiology of community-acquired pneumonia in children in primary healthcare settings. Scand J Infect Dis 2000;32:399-402.
4. Arnon S, Litmanovitz I, Regev R, Lis M, Shainkin-Kestenbaum R, Dolfin T. Serum amyloid A protein in the early detection of late-onset bacterial sepsis in preterm infants. J Perinat Med 2002;30:329-32.
5. Yoon BH, Romero R, Shim JY, Shim SS, Kim CJ, Jun JK. C-reactive protein in umbilical cord blood: a simple and widely available clinical method to assess the risk of amniotic fluid infection and funisitis. J Matern Fetal Neonatal Med 2003;14:85-90.
6. Santana Reyes C, Garcia-Munoz F, Reyes D, Gonzalez G, Dominguez C, Domenech E. Role of cytokines (interleukin-1beta, 6, 8, tumour necrosis factor-alpha, and soluble receptor of interleukin-2) and C-reactive protein in the diagnosis of neonatal sepsis. Acta Paediatr 2003;92:221-7.
7. Madan A, Adams MM, Philip AG. Frequency and timing of symptoms in infants screened for sepsis:effectiveness of a sepsis-screening pathway. Clin Pediatr (Phila) 2003;42:11-8.
8. Chiesa C, Pellegrini G, Panero A, Osborn JF, Signore F, Assumma M, et al. C-reactive protein, interleukin-6, and procalcitonin in the immediate postnatal period: influence of illness severity, risk status, antenatal and perinatal complications, and infection. Clin Chem 2003;49:60-8.
9. Chiesa C, Panero A, Osborn JF, Simonetti AF, Pacifico L. Diagnosis of Neonatal Sepsis: A Clinical and Laboratory Challenge. Clin Chem 2004;50:279-87.
10. Vazzalwar R, Pina-Rodrigues E, Puppala,Angst DB, Schweig L. Procalcitonin as a screening test for late-onset sepsis in preterm very low birth weight infants. J Perinatol 2005;25(6):397-402.
11. Weitkamp JH, Aschner JL. Diagnostic Use of C-Reactive Protein (CRP) in Assessment of Neonatal Sepsis. Neoreview 2005;6:508-15.
12. Joram N, Boscher C, Denizot S, Loubersac V, Winer N, Roze JC, et al. Umbilical cord blood procalcitonin and C reactive protein concentrations as markers for early diagnosis of very early onset neonatal infection. Arch Dis Child Fetal Neonatal Ed 2006;91(1):65-6.
13. Flegar-Meštrić Z, Perkov S, Šiftar Z. Evaluation of immunoturbidimetric full-range method for C-reactive protein. Farm Vestn 2004;55:372-3.