Introduction
Pulmonary tuberculosis is a disease characterized by inflammation of lung parenchyma and is caused by the intracellular bacterium Mycobacterium (M.) tuberculosis. There are some two billion people with latent M. tuberculosis infection (LTBI) worldwide (1). As a long time may elapse from infection to disease manifestation, identification of those infected before the initial disease development is of utmost importance to prevent it to flare up by timely prophylaxis. Tuberculin skin test (TST) as the gold standard, and in vitro determination of interferon gamma (IFN-) as one of recent diagnostic methods (2,3) (including history, data on contact with a tuberculosis patient, clinical picture, and chest x-ray) are crucial tests used to detect M. tuberculosis infection. Side effects of antituberculotics in patients with active tuberculosis and individuals with LTBI are monitored by determination of complete blood count, platelet count and liver function tests (4), whereas longitudinal determination of C-reactive protein (CRP) concentration can be used as an indicator of disease activity (5).
CRP is an established marker of acute inflammation, and its serum concentration is frequently determined to assess the grade of systemic inflammation (6), e.g., in rheumatic (7) or intestinal (8) diseases, or to verify bacterial etiology of inflammation such as pneumonia in adults (9) and children (10) alike. Recently, the immunoturbidimetric method on latex particles has been used to determine very low CRP concentrations. The method has improved analytical sensitivity in determining serum concentration of high-sensitive CRP (hsCRP) to 0.1 mg/L, thus enabling the use of hsCRP concentration as a prognostic marker of chronic inflammation in patients with cardiovascular disease (11), diabetes mellitus (12, 13) and asthma (14, 15).
CRP as an acute phase protein is involved in the regulation of the complement system (16). In the last few years, research into the role of complement system has been revived because of its role not only in infection, inflammation or allergic reaction but also in the apoptotic cell clearance (17) and development of autoimmune disorders (18), and for its potential therapeutic use (19). M. tuberculosis can also activate complement system via C1 human monocyte receptor (20).
The objective of the present study was to assess changes in the concentration of hsCRP, C3 and C4 in children with latent M. tuberculosis infection. Concentrations of hsCRP as well as complement components C3 and C4 were determined before drug administration and after a two-month period during which drugs were administered on a daily basis.
Subjects and methods
Subjects
The study included 79 subjects aged 2.5 months to 18 years, referred between October 2005 and January 2007 to Srebrnjak Children’s Hospital in Zagreb for systematic examination or detection of possible M. tuberculosis infection. The subjects were divided into three groups according to diagnosis:
1. subjects with LTBI, with a positive history of close contact with a tuberculosis patient and purified protein derivative (PPD) hyperresponsiveness (N = 26, aged 1–18 years) and were administered isoniazid for prophylaxis;
2. subjects with lung tuberculosis (N = 18, aged 2.5 months to 18 years), diagnosed on the basis of clinical picture, positive PPD test finding, specific tuberculotic lesions on chest x-ray, and microbiological finding of M. tuberculosis in sputum, bronchial aspirate or gastric lavage. Patients with tuberculosis received therapy consisting of four antituberculotics (isoniazid, rifampicin, ethambutol and pyrazinamide);
3. control group, clinically healthy subjects (N = 35, aged 4–18 years), referred for systematic examination, with biochemistry and hematology parameters within the reference range for age.
Blood sampling was performed between 8.00 and 12.00 a.m. At two months of therapy, repeat blood samples were collected in LTBI subjects and tuberculosis patients. The investigation was approved by the hospital ethics committee and a written informed consent was obtained from parents.
Methods
Determination of hsCRP, C3 and C4 concentrations
The concentrations of hsCRP (immunoturbidimetric method on latex particles), and C3 and C4 complement components (immunoturbidimetric method) were determined on an Olympus AU 400 biochemistry analyzer (Olympus, Tokyo, Japan), using Olympus System Reagents (Olympus Diagnostica, Hamburg, Germany).
Statistical analysis
Data were stored and prepared for statistical analysis by use of the Microsoft Office Excel 2000 software (Microsoft, USA). On data processing, MedCalc (Medisoftware, Mariakerke, Belgium) (21) was used. The variables with normal distribution were described by arithmetic mean (x) and standard deviation (SD), and those not showing normal distribution were presented by median (M) and interquartile range (IQR). Paired Student’s t-test and Wilcoxon test were used for comparison of dependent variables (for normal distribution and asymmetric distribution, respectively). ANOVA test was used for comparison of multiple independent groups, and non-parametric Kruskal-Wallis test for distributions that were not normal. The values of P < 0.05 were considered statistically significant. ROC (Receiver Operating Characteristic) analysis was used to determine optimal cut-off values, area under the ROC curve (AUC), specificity, sensitivity and predictive values.
Results
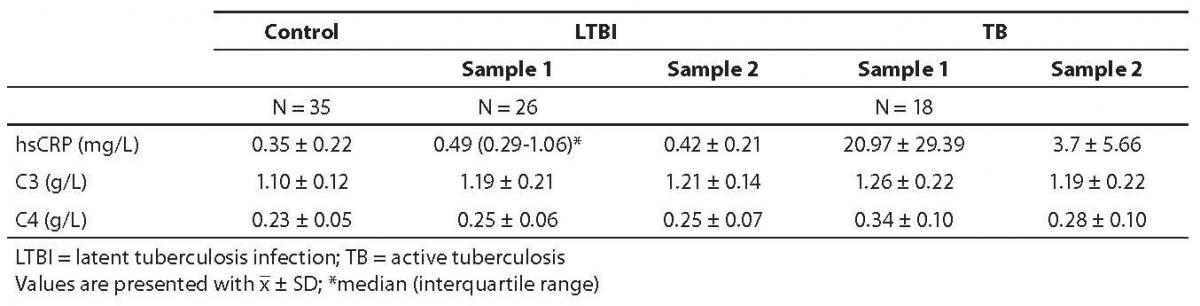
Results of patients and controls are shown in Table 1. Statistical analysis indicated that there was normal distribution for all analytes, except for hsCRP in LTBI group, sample 1. ANOVA test for C3 and C4 showed a statistically significant difference between study groups (P = 0.007 and P < 0.001, respectively). Before prophylactic isoniazid therapy induction (sample 1), the concentration of hsCRP, C3 and C4 was significantly higher in LTBI subjects in comparison with control group (Table 2). Also, in the group of tuberculosis patients, pretherapeutic concentration of hsCRP, C3 and C4 (sample 1) was statistically significantly higher than the concentration recorded in control group.
Table 1. Concentrations of hsCRP, C3 and C4 in control group, LTBI group and tuberculosis group of children before (sample 1) and after (sample 2) therapy.
Table 2. Statistical significance of differences in hsCRP, C3 and C4 values between control subjects and subjects with LTBI and tuberculosis (ANOVA test and Kruskal-Wallis test)
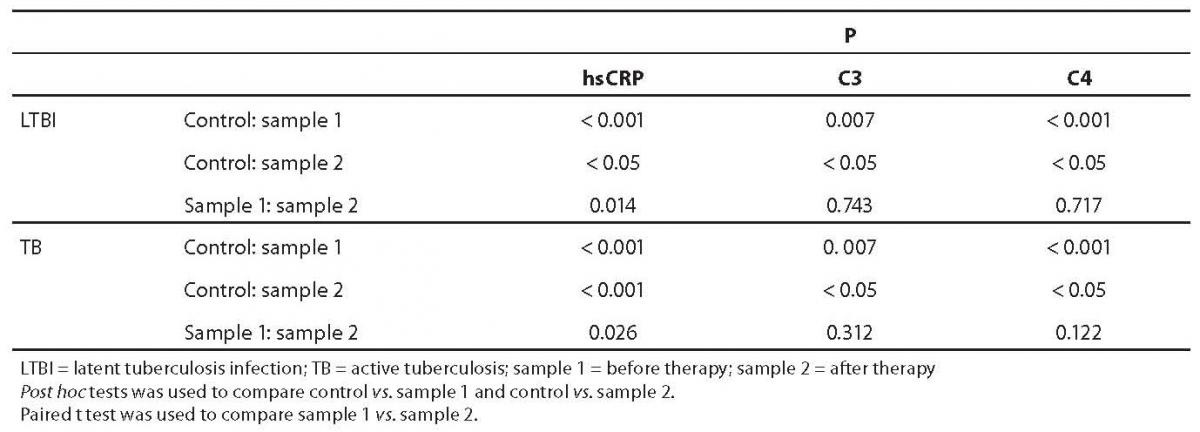
After prophylactic therapy the concentration of hsCRP in LTBI (sample 2) was lower than before isoniazid administration. Also, after two-month therapy (TB group, sample 2), the concentration of hsCRP decreased significantly (but remained statistically significantly higher in comparison with control group). Post hoc test indicated differences between LTBI sample 1 vs. control group and tuberculosis sample 1 vs. control group. Kruskal-Wallis test for hsCRP indicated significant differences between study groups (P < 0.001). There was no significant difference between pretherapeutic and post-therapeutic values of C3 and C4 either in LTBI or in tuberculosis group. However, a significant difference between pretherapeutic and post-therapeutic values was found for hsCRP values in both LTBI (P = 0.014) and tuberculosis (P = 0.026) groups.
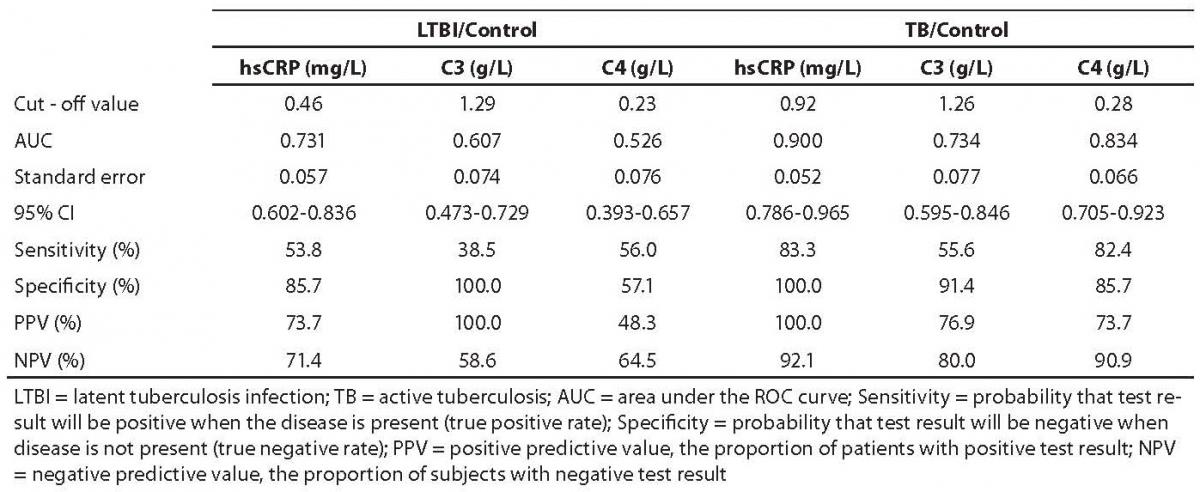
ROC curves were elaborated in LTBI and tuberculosis groups at pretherapeutic values (Table 3). In tuberculosis group, only hsCRP showed a statistically significantly greater area under curve (AUC) (P = 0.037) as compared with control group. In LTBI group, no statistically significant difference was recorded for any of the study parameters (P > 0.05).
Table 3. ROC analysis of pretherapeutic values of hsCRP, C3 and C4 in LTBI subjects and tuberculosis patients
Discussion
Study results indicated the concentrations of hsCRP, C3 and C4 to be statistically significantly higher in children with LTBI (before prophylaxis) and children with tuberculosis (pre-therapeutically and after 2-month antituberculotic therapy) as compared with the control group of healthy children. Also, the values of hsCRP were significantly lower after two months of therapy (as compared with pretherapeutic values) in both LTBI and tuberculosis groups.
As early as 1989, Bajaj et al. pointed to the possible use of CRP in the assessment of tuberculosis activity (5). However, literature data on the determination of CRP concentration in serum of tuberculosis patients are contradictory. Some authors describe patients with active tuberculosis without increase in CRP concentration (22), whereas others report on patients with elevated CRP concentration (23,24). Also, our results indicated that monitoring of hsCRP concentration could be used in the follow up of patients with tuberculosis and LTBI. In LTBI children, determination of CRP concentration by a high sensitivity method (hsCRP) is of special value because it may point to moderate inflammatory lesions that cannot be identified by the methods of lower sensitivity. In this case, borderline values discriminating healthy subjects from affected ones are significantly lower (15). We found no reports on hsCRP concentration in children with M. tuberculosis infection in the available literature.
Complement component C3 can activate alternative pathway (20,25), whereby the CR3 complement receptor on the macrophage surface appears to be involved in mediating M. tuberculosis binding to macrophages but not intracellular replication of M. tuberculosis (26,27). According to Stokes et al., the intensity of M. tuberculosis binding to macrophages depends on the macrophage phenotype (28). Dubaniewicz et al. describe elevated concentration of C3 but not of C4 in patients with active tuberculosis (27). In their study, the levels of C3 and C4 were not changed in patients with inactive tuberculosis. In our study, both C3 and C4 were increased in subjects with LTBI and in patients with tuberculosis.
The fact that specificity was lower than sensitivity indicates that healthy subjects (i.e. true negative rates) could be better distinguished on the basis of optimal cut-off values of selected analytes.
In most cases of initial tuberculosis infection in children there are no symptoms and no complications (29). The lesions of lung parenchyma are not visible on chest x-ray, while lymph nodes remain normal in size. The child only shows positive tuberculin skin test. These preliminary results have suggested that latent chronic inflammation is not probable in children with positive PPD, and with hsCRP, C3 and C4 concentration less than cut-off values. Definitive decision on the prophylaxis will be made by physician.
This is, to our knowledge, the first report on hsCRP in children with LTBI. The limitation of the present study was the small number of study subjects, to which the inadequate statistical significance of difference on ROC curve analysis could have also been ascribed. However, may the results of this study be confirmed in an independent study including a greater number of subjects, hsCRP could be considered a useful marker in the follow up of LTBI patients, to evaluate the response to isoniazid prophylaxis and the level of the disease activity.
Notes
Potential conflict of interest
None declared
References
1. World Health Organization. Global tuberculosis control: surveilleance, planning, financing. WHO Report 2005. Geneva, 2005.
2. Lalvani A, Pathan AA, McShane H, Wilkinson RJ, Latif M, Conlon CP, et al. Rapid detection of Mycobacterium tuberculosis infection by enumeration of antigen-specific T-cells. Am J Respir Crit Care Med 2001;163:824-8.
3. Mazurek GH, LoBue PA, Daley CL, Bernardo J, Lardizabal AA, Bishai WR et al. Comparison of a whole-blood interferon γ assay with tuberculin skin testing for detecting latent Mycobacterium tuberculosis infection. JAMA 2001;286:1740-7.
4. Targeted Tuberculin Testing and Treatment of Latent Tuberculosis Infection. ATS/CDC Statement Committee on Latent Tuberculosis Infection Membership List, June 2000. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr4906a1.htm. Accessed December 27th 2007.
5. Bajaj G, Rattan A, Ahmad P. Prognostic value of C-reactive protein i tuberculosis. Indian Pediatr 1989;26:1010-3.
6. Pepys MB, Baltz MC. Acute phase proteins with special reference to C-reactive protein and related proteins (pentaxins) and serum amyloid A protein. Adv Immunol 1983;34:141-212.
7. Emery P, Luqmani R. The validity of surrogate markers in rheumatic disease. Br J Rheumatol 1993;32:Suppl 3:3-8.
8. Walker-Smith JA. Chronic inflammatory bowel disease in children: a complex problem in management. Postgrad Med J 2000;76:469-72.
9. Lagerstrom F, Engfeldt P, Holmberg H. C-reactive protein in diagnosis of community-acquired pneumonia in adult patients in primary care. Scand J Infect Dis 2006;38:964-9.
10. Korppi M. Non-specific host response markers in the differentiation between pneumococcal and viral pneumonia: what is the most accurate combination? Pediatr Int 2004;46:545-50.
11. Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation 2003;107:363-9.
12. Coulon J, Willems D, Dorchy H. Increase in C-reactive protein plasma levels during diabetes in infants and young adults. Presse Med 2005;34:89-93.
13. Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001;286:327-34.
14. Takemura M, Matsumoto H, Niimi A, Ueda T, Matsuoka H, Yamaguchi M, et al. High sensitivity C-reactive protein in asthma. Eur Respir J 2006;27:908-12.
16. Galez D, Dodig S, Raos M, Nogalo B. CRP in children with asthma and allergic rhinitis. Biochemia Medica 2006;16(2):163-9.
16. Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest 2003:111:1805-12.
17. Botto M. Links between complement deficiency and apoptosis. Arthritis Res 2001;3:207-10.
18. Boackle SA, Holers VM. Role of complement in the development of autoimmunity. Curr Dir Autoimmun 2003;6:154-6.
19. Boackle SA. Complement and autoimmunity. Biomed Pharmacother 2003;57:269-73.
20. Schlesinger LS, Bellinger-Kawahara CG, Payne NR, Horwitz MA. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J Immunol 1990;144:2771-80.
22. Ito KM, Yoshiyama T, Wada M, Ogata H. C-reactive protein in patients with bacteriological positive lung tuberculosis. Kekkaku 2004;79:309-11. Available at; http://www.ncbi.nlm.nih.gov/pubmed/ Accessed August 7th 2007.
23. Peroš-Golubičić T. C-reactive protein in sarcoidosis. Acta Med Iugosl 1991;45:169-74.
24. Koyanagi A, Kuffö, Gresely L, Shenkin A, Cuevas LE. Relationships between serum concentrations of C-reactive protein and micronutrients in patients with tuberculosis. Ann Trop Med Parasitol 2004;98:391-9.
25. Mueller-Ortiz SL, Wanger AR, Norris SJ. Mycobacterial protein HbhA binds human complement component C3. Infect Immun 2001; 69: 7501-11.
26. Velasco-Velazquez MA, Barrera D, Gonzalez-Arenas A, Rosales C, Agramonte-Hevia J. Macrophage – Mycobacterium tuberculosis interactions: role of complement receptor 3. Microb Pathog 2003;35:125-31.
27. Dubaniewicz A, Sztaba-Kania M, Hoppe A. Analysis of some immunological parameters in pulmonary tuberculosis. Pol Merkur Lekarski 2004;16:123-7.
28. Stokes RW, Haidl ID, Jefferies WA, Speert DP. Mycobacteria – macrophage interactions. Macrophage phenotype determines the nonopsonic binding of Mycobacterium tuberculosis to murine macrophages. J Immunol 1993;151:7067-76.
29. Starke JR. Tuberculosis in children. Semin Respir Crit Care Med 2004; 25:353-64.