Introduction
The concept of D-dimer measurement as an indicator of in vivo coagulation activation has been introduced more than two decades ago (1,2). Today, measurement of D-dimer has become an essential element in the diagnostics of deep vein thrombosis and pulmonary embolism (3-6).
It has been observed that the numerical assay results of different D-dimer assays vary considerably (7). This is mainly caused by the heterogeneity of fibrin degradation products in patient samples (8), as well as the specificity of the different antibodies used in these assays (9). D-dimer assays show different reactivity to different kinds of fibrin derivatives, such as high or low molecular weight fibrin derivatives, or cross-reactivity with non-cross-linked fibrinogen- and fibrin degradation products. Lack of standardization and different types of calibrators used by the manufacturers are additional issues that affect the assay results (10).
In an attempt to standardize D-dimer assays various standards such as purified D-dimer, whole blood lysate and X-oligomers have been used, but failed (11,12). Therefore, harmonization of D-dimer assays by using patient pooled plasma with a high D-dimer concentration as a reference preparation seems to be another option (13).
This approach to harmonization of quantitative D-dimer results by the use of patient pooled plasma with a high D-dimer value (14) was used at the University Medical Centre Ljubljana, where D-dimer assays are performed in several laboratories affiliated to different clinical departments. It was presumed that the harmonization procedure would increase the comparability of D-dimer results obtained in different laboratories of the same medical institution.
Materials and methods
Plasma preparation
For preparation of normal pooled plasma, blood was collected from 25 apparently healthy volunteers, mainly laboratory staff and students. Blood from the cubital vein was collected into 5 mL vacuum tubes containing 0.5 mL of 0.109 mol/L sodium citrate (Becton Dickinson, Vacutainer System Europe, Heidelberg, Germany), thoroughly mixed with the anticoagulant, placed immediately in ice water and centrifuged at 4 °C and 2000 x g for 30 minutes within 4 hours of venepuncture. Plasma was removed, pooled, aliquotted, frozen in liquid nitrogen and stored at -70 oC until analysis. For preparation of harmonization samples, stored plasma samples from 25 patients with venous thromboembolic disease and high D-dimer concentration (well above the cut-off level) were thawed and pooled (patient pooled plasma). In these samples D-dimer was determined by Auto-Dimer (Biopool Trinity Biotech, Umea, Sweden). A set of five harmonization plasma samples with increasing concentrations of D-dimer were prepared by mixing normal pooled plasma with increasing parts (1, 2, 3, 8 and 14.7) of patient pooled plasma (harmonization samples A-E), aliquotted and frozen in liquid nitrogen. Mixing of 6.35 mL of normal pooled plasma with 0.15 mL of patient pooled plasma (sample A) was stated as one part. For validation of the harmonization procedure, 15 stored plasma samples (others than those as for harmonization samples) with a different concentration of D-dimer from patients with venous thromboembolism (validation samples V01-V15) were utilized. Frozen aliquots of normal pooled plasma, harmonization samples A-E and validation samples V01-V15 were transported to participating laboratories of the University Medical Centre Ljubljana. After thawing, samples were analysed within two hours. All patient plasma samples used for harmonization and validation were obtained according to the same protocol as described for normal pooled plasma.
Methods
Five laboratories from the University Medical Centre Ljubljana participated in the study. Five different quantitative D-dimer assays were used as routinely or for this exercise only: Vidas D-dimer Exclusion on a Vidas analyser (both bioMerieux, Durham, North Carolina, USA,Laboratory 1), Auto-Dimer (Biopool Trinity Biotech, Umea, Sweden, Laboratory 1) and D-dimer Plus (Dade Behring, Marburg/Lahn, Germany) on a Behring Coagulation Timer (BCT, Dade Behring, Marburg/Lahn, Germany, Laboratory 1 and Laboratory 4) and on a Behring Coagulation System (BCS, Dade Behring, Marburg/Lahn, Germany, Laboratory 2), Acute Care™ D-Dimer on a Stratus CS (both Dade Behring, Marburg/Lahn, Germany, Laboratory 1 and Laboratory 3), Hemosil D-dimer test on an ACL 7000 (Laboratory 4) and an ACL 9000 (all Instrumentation Laboratory, Warrington, United Kingdom, Laboratory 5).
Harmonization and validation procedure and other calculations
All laboratories tested normal pooled plasma and harmonization samples A-E in triplicate. Assay-specific mean values were calculated (Table 1) and corrected for the contribution of the D-dimer in normal pool plasma. Regresion analysis was utilized to calculate assay-specific regression lines and an overall reference regression line through assay-specific median values and overall median value for each sample, respectively (Table 2).
Validation samples V01-V15 were tested in duplicate and reported as means. Results were harmonized as described previously (14) according to the slope (a) and intercept (b) of the assay-specific regression line (m) and reference regression line of the overall median values (h) according to the formula:
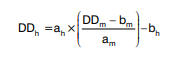
For example, the harmonized value of sample V08 determined with Vidas D-dimer Exclusion (Table 3) was calculated as:
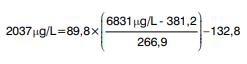
where 6831 mg/L was the raw result for sample V08 obtained with Vidas D-dimer Exclusion and 2037 mg/L was calculated as the harmonized result.
The variability of the results was expressed in percent as the coefficient of variation. Excel 97 (Microsoft Corporation, Orlando, Florida, USA) was used for all calculations.
Results
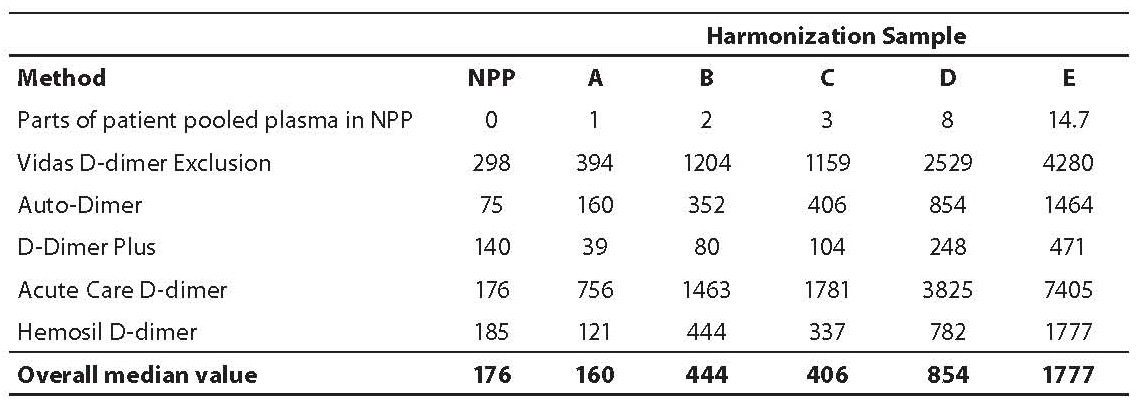
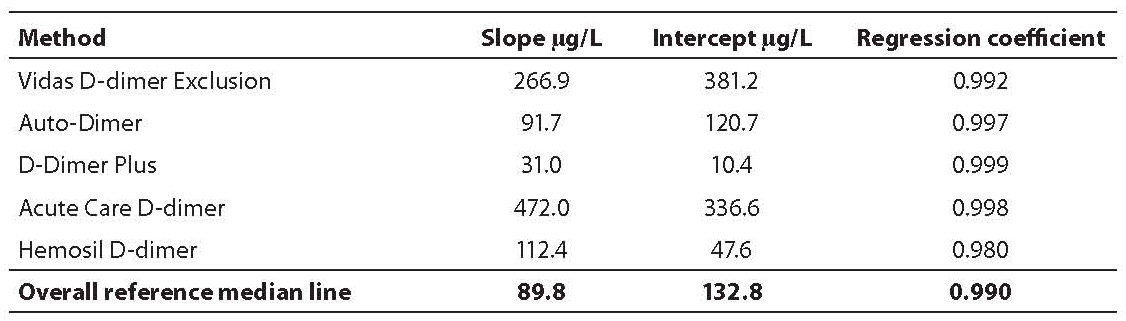
Mean D-dimer concentrations (µg/L) in harmonization samples A-E for each assay obtained are shown in Table 1. Values of normal pool plasma obtained by each assay, which were deducted from raw values of samples A-E are also shown in Table 1. In Table 2 the slope, intercept and regression coefficients of the linear regression analysis through the assay specific values and the overall median line are shown.
Table 1. Assay specific mean D-dimer levels (µg/L) in normal pooled plasma (NPP) and in harmonization samples A–E.
Tablica 2. The slope, intercept and regression coefficients of the linear regression analysis through the assay-specific values and the overall reference median line
The highest D-dimer values were observed with Acute Care™ D-Dimer on Stratus CS, while approximately 18-26 times lower values were determined with D-dimer Plus on a BCT or BCS, with other D-dimer concentrations in between.
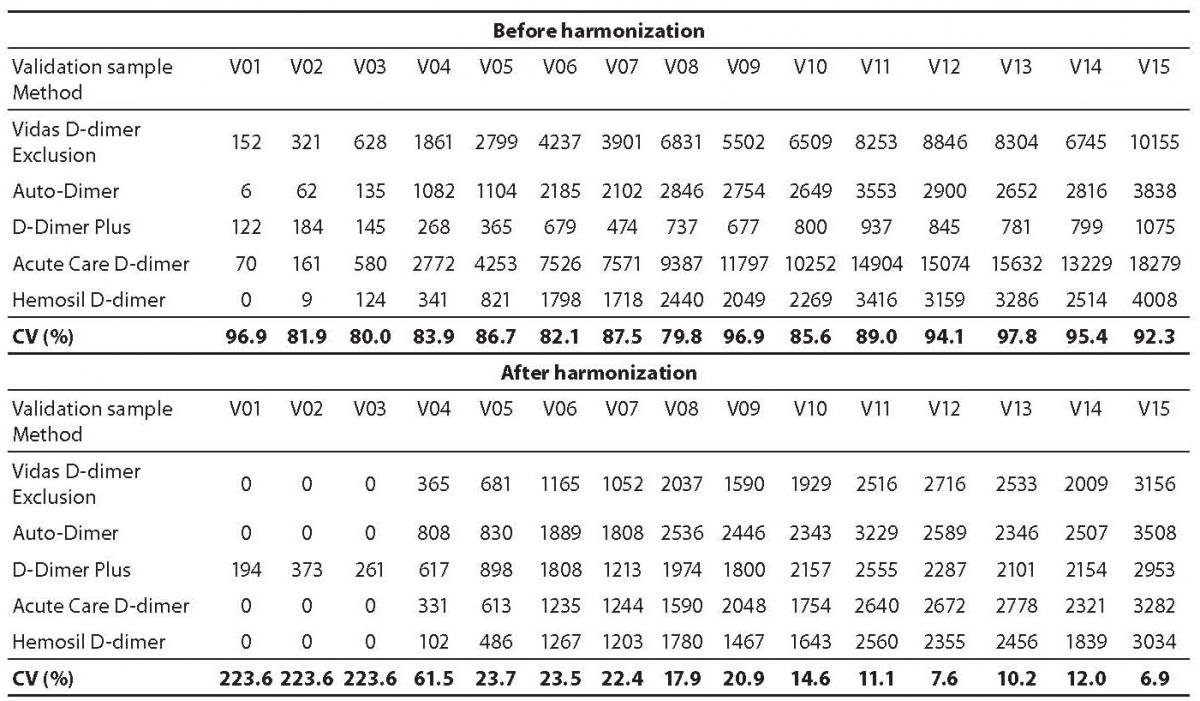
As expected, results of D-dimer measurements in validation samples V01-V15 with different assays showed considerable variability before harmonization. In general, harmonization resulted in significantly reduced variability in D-dimer concentration in validation plasma samples V01-V15. For all validation samples the mean coefficient of variation decreased from 89% before harmonization to 60% after harmonization. A greater reduction in variability from 89% to 19% (both mean values) was observed for validation samples V04-V15 with high D-dimer concentrations. On the other hand, for 3 validation samples V01-V03 with normal and D-dimer concentrations around cut-off the coefficients of variation even increased from a mean value of 86% before harmonization to mean value of 224% after harmonization (Table 3).
Table 3. D-dimer levels (µg/L) in validation samples V01-V15 obtained by different assays before and after harmonization. Coefficients of variation (CV) are shown for each validation sample.
Discussion
The present study showed, that harmonization of results of quantitative D-dimer assays with patient pooled plasma was possible, but was limited to high D-dimer values. In the range of D-dimer around the cut-off, harmonization did not decrease the variability of the results.
The initiative for harmonization was provoked by the fact that in the same medical institution several D-dimer assays are used. Since the results obtained with these assays are not comparable, management of patients might not be optimal if physicians are not aware of the differences between different assays and therefore the different cut-off values. Moreover, exchange of D-dimer results between different hospitals is hampered. It is recommended that due to differences in monoclonal antibodies, assay technologies, and calibration, the results of different D-dimer assays should not be used in clinical studies, and the assay used should be clearly stated not only in scientific publications but also in clinical routine, to allow appropriate interpretation of D-dimer concentration measured (10).
Since D-dimer antigen is not a homogeneous analyte, but represents a conglomerate of fibrin derivatives of different molecular sizes and structures (15), standardization of D-dimer assays is difficult (13,16,17). Currently, results of D-dimer measurements are based on assay-specific reference curves, the calibrators being selected by the respective manufacturer. Numerical results are reported as D-dimer concentration, based on calibration with purified D-dimer, or as fibrinogen-equivalent units (FEU), based on the amount of purified fibrinogen used for the preparation of a cross-linked fibrin clot, which is then degraded by plasmin and used as a calibrator. The third kind of calibrators used by some manufacturers are based on pooled plasma from patients with elevated D-dimer concentration (10). Since the different approaches to calibration lead to variable results of D-dimer concentration, a consensus was reached that a common calibrator is needed. This calibrator should reflect the heterogeneity of D-dimer antigen-containing compounds found in clinical blood samples, a goal that has not yet been achieved (18).
Another approach to comparable D-dimer values obtained with different assays is harmonization by the use of patient pooled plasma (13,17). Harmonization is based on mathematical transformation of regression lines through the assay-specific values of a set of plasma samples with different D-dimer concentration to a reference regression line through the overall mean values of all the D-dimer assays being harmonized (14,19). The advantage of this approach is that all the different kinds of fibrin degradation products will be present in the patient pooled plasma, and a limitation is that an individual patient or specific patient groups (e.g. patients with deep vein thrombosis or patients with disseminated intravascular coagulation) may behave differently. Because there is no reference assay for D-dimer available, the amount of patient pooled plasma added to normal pooled plasma is used as an independent variable. Assay-specific values used in this harmonization model reflect the response of the different assays to the kind of D-dimer in the patient pooled plasma used. If a large number of laboratories are participating in the harmonization, then the estimated assay-specific consensus values are a reliable approximation to the so-called “analytical true values” for these samples.
In the present study harmonization of test results of different D-dimer assays was performed as published (14). Compared to the harmonization procedure published (14), which lead to a reduction of the variability from about 75% to 5.5%, in our study reduction of variability was much smaller (from about 89% to about 60%) for all validation samples, but was considerably better for samples with high D-dimer concentration (V04-V15) (reduction from about 89% to 19%). For samples with D-dimer concentration around and below the cut-off (V01-V03), the coefficient of variation even increased after harmonization (from about 86% to about 224%). In the published study (14) over 500 laboratories participated using in a total of 20 different assays. Although only the seven most frequently used assays were used in the set-up of the harmonization model, the number of results was considerably greater compared to our study in which only five laboratories participated. Although the harmonization procedure performed in our study could not be adopted to report results from different laboratories, it represented an exercise on the (non)comparability of the D-dimer assays results used in our medical institution.
Conclusion
To conclude, the harmonization procedure adopted considerably improved comparability between different D-dimer assays in samples with high D-dimer concentration. However, for samples with normal D-dimer and D-dimer close to the cut-off value no improvement in variability was observed. This could partly be attributed to the small number of participating laboratories, but also to the small number of harmonization samples in the normal and around the cut-off ranges. Since D-dimer values around the cut-off are critical for exclusion of deep vein thrombosis or pulmonary embolism, this harmonization procedure could not be adopted to report results from different laboratories.
Acknowledgements
The authors are indebted to laboratory heads Katarina Lenart, Nada Snoj, Alenka Trampuš Bakija and Jana Kralj and technicians from these laboratories for excellent technical assistance, and to the Slovenian representative of Dade Behring, and companies Dipros and Mikro+Polo for providing reagents and analysers.


