Introduction
Type 2 diabetic patients exhibit dyslipidemia with high triglyceride and low high density lipoprotein (HDL-cholesterol) concentration which are the underlying pathology behind cardiovascular disease (1,2). Numerous population studies have shown an association between plasma lipid levels and coronary artery disease risk (3,4). Moreover, epidemiological studies suggest that the risk of developing coronary heart disease for patient with diabetes mellitus is 2–4 times higher than their counterparts without diabetes (1). Some experimental studies suggested that accumulation of cholesterol in islets causes to reduced glucose-stimulated insulin secretion (GSIS), and impaired glucose tolerance in mice. Therefore elevated cholesterol levels may be a risk factor for glucose intolerance and diabetes (5).
Adenosine binding cassette transporter proteins 1 (ABCA1) plays a role in cholesterol metabolism, especially HDL-cholesterol. Previous investigations showed that homozygous mutations in the ABCA1 gene cause Tangier disease and heterozygosity causes for familial hypoalphalipoproteinemia. There are multiple mechanisms by which HDL-cholesterol can be atheroprotective, it is clear that the relative activity of ABCA1 plays a major role. Genetic and molecular biology studies have suggested that low plasma HDL-cholesterol concentration in many individuals reflect an impaired ABCA1 pathway, which would also promote the accumulation of cholesterol in tissue macrophages. Albrecht et al. showed that ABCA1 gene expression was significantly elevated in atherosclerotic plaques (6). Porchay et al. suggested that ABCA1 gene polymorphisms modulate HDL-cholesterol concentrations in an interaction with body mass index (BMI), and thus, they might influence cardiovascular risk in the general population (7). In addition, ABCA1 R230C polymorphism was shown to be associated with a 4-fold occurrence of diabetes and it may have an important role in maintaining glucose-mediated insulin secretion (8). Salinas et al. reported that the absence of ABCA1 led to cholesterol accumulation within the beta cell plasma membrane, suggesting that ABCA1 may be not only a determinant of HDL-cholesterol but also a link among diabetes, metabolic syndrome and atherosclerosis (9). In another study, the authors have demonstrated that high glucose can suppress the mRNA expression of ABCA1 in mouse primary peritoneal macrophages (10).
There were fewer investigations about the role of ABCA1 C69T polymorphism (rs1800977) in lipid related disorders such as diabetes, cardiovascular disease etc. According to these knowledge, we aimed to investigate the relationship between ABCA1 C69T gene polymorphisms and lipid concentrations in Turkish type 2 diabetic patients.
Materials and methods
Study design and subjects
Our investigation was a case-control study.
The patient group consisted of 107 patients (median = 56 years; min-max = 25-85 years; 71 females and 36 males) with type 2 diabetes. The patients were recruited from Department of Internal Medicine, Haseki Training and Research Hospital between in the period from 2009 to 2011. Patients were diagnosed with diabetes mellitus based on the fasting blood glucose concentration (> 7 mmol/L) (11). Systemic arterial hypertension was considered to be present if the SBP was > 130 mm Hg and/or DPB was > 90 mm Hg.
The control group was selected from patients attending the general surgery and orthopedic clinics of the same hospital and who were treated for trauma. BMI (weight in kilograms divided by the square of the height in meters) values were calculated retrospectively and categorized according to World Health Organization recommendations (12). The smoking status of an individual was assigned ‘yes’ if they were smoking currently or had given up < 3 months previously. The control group was selected from patients without type 2 diabetes and with negative family history of diabetes mellitus. It consisted of 50 individuals (median = 49 years; min-max = 29-85 years; 17 females and 33 males).
This study was approved by the Ethical Committee of Istanbul University, The Istanbul Faculty of Medicine. All of participants, after giving written informed consent, completed a structured questionnaire in order to collect demographic data.
Determination of biochemical parameters
After overnight fasting, blood samples were drawn in plain tubes (Vacuette, Greiner Labor technik, Germany). One plain tube was used for each subject. The samples were centrifuged for 10 min at 1,500 × gat room temperature, followed by the removal of serum. Serum was stored at -20 °C, until concentrations of triglycerides, total cholesterol and fasting blood glucose were determined by using Hitachi 717 autoanalyzer (Tokyo, Japan). Concentrations of HDL-cholesterol were measured subsequently after precipitation of ApoB-containing lipoproteins with phosphotungstic acid/MgC12. LDL-C concentration was calculated using the Friedewald formula when the triglyceride concentration was < 4 mmol/L; otherwise, LDL-C concentration was measured directly using the Hitachi 717 autoanalyzer (Tokyo, Japan) (13,14).
Isolation of DNA
Blood specimens were collected in tubes containing EDTA (Vacuette, Greiner Labor technik, Germany) and DNA was prepared from the leukocyte pellets by sodium dodecyl sulphate lysis (Sigma Aldrich, Taufkirchen, Germany), ammonium acetate (Sigma Aldrich, Taufkirchen, Germany) extraction and ethanol (Sigma Aldrich, Taufkirchen, Germany) precipitation (15). One tube containing EDTA was used for each subject. DNA samples were stored at +4 °C until PCR application.
Determination of ABCA1 C69T gene polymorphism
Gene polymorphisms were determined using following PCR-RFLP method (16). Template DNA (0.5-1.0 mg) was used in a PCR under stringent conditions to avoid the possibility of false positives for C69T genotyping. Reactions were performed with 10 pmol of each primer (Invitrogen, Grand Island, USA): forward primer 5’ CAG CGC TTC CCG CGC GTC TTA 3’, reverse primer 5’CCA CTC ACT CTC GTC CGC AAT TAC 3’ in final volume of 25 mL containing 1.5 mM MgCl2, 50 mM KCl, 10 mM Tris-HCl (pH 8.4), 100 mmol/L of each dNTP (Invitrogen, Grand Island, USA) and 1 unit Taq Polymerase (Invitrogen, Grand Island, USA). Amplification was carried out in a DNA Thermal Cycler (Applied Biosystems, Carlsbad, USA) for 33 cycles with denaturation steps at 94 °C for 1 minute, annealing at 60 °C for 1 minute and extension at 72°C for 2 minutes. PCR products were separated on 2% agarose gel and DNA was visualized by ethidium bromide (Invitrogen, Grand Island, USA) staining. Presence of the polymorphisms was determined by enzymatic digestion of the initial PCR product with BsmAI (Invitrogen, Grand Island, USA) at 37 °C for 2h. Three genotypes could be determined after electrophoresis: genotype CC (345 bp band), genotype TT (310 bp band) and genotype CT (both bands).
Statistical analysis
Allele and genotype frequencies were determined by direct counting. Categorical variables such as genotypes and alleles were compared using Chi-Square (χ2) test and z-test. Differences in continuous variables between carriers and control subjects were tested using Student’s t test, except for creatinine, which was not normally distributed. Differences in creatinine and continuous outcome variables between carriers and control subjects were tested using the nonparametric Mann-Whitney U test (17).
Statistical analyses were performed with SPSS for Windows standard version 7.5 software (SPSS Inc, Chicago, USA). A P value of 0.05 was accepted as the threshold for defining statistical significance.
Results
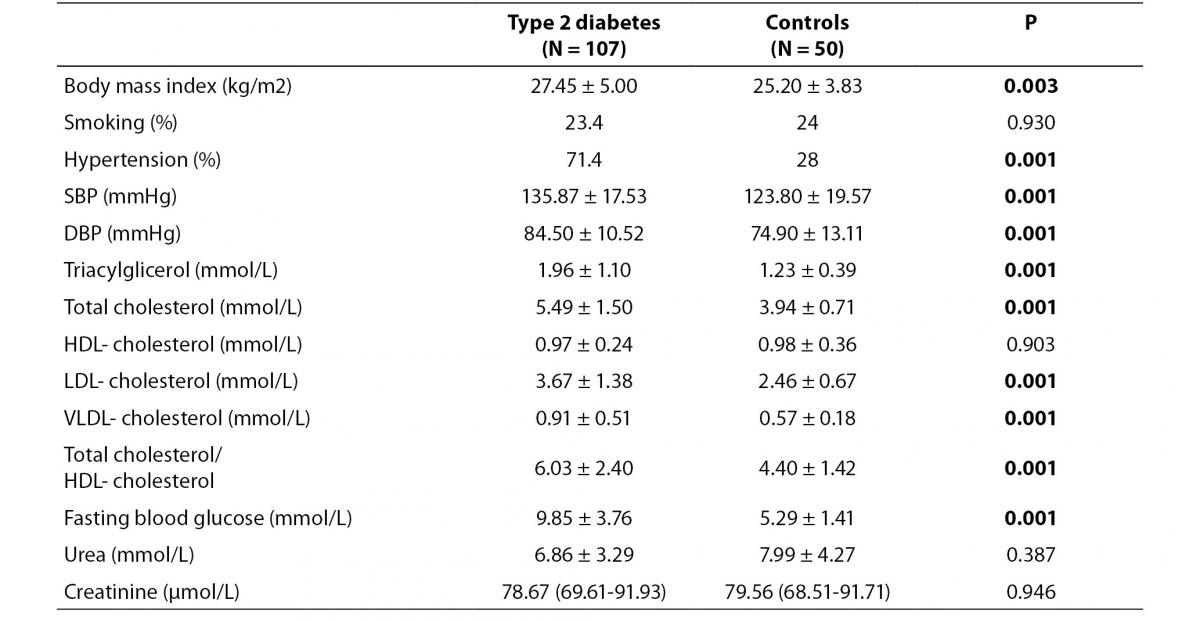
Demographic characteristics are summarized in table 1. Total cholesterol (P = 0.001), triglycerides (P = 0.001), LDL-cholesterol (P = 0.001), VLDL-cholesterol (P = 0.001), systolic blood pressure (SBP) (P = 0.001), diastolic blood pressure (DBP) (P = 0.001), fasting blood glucose (P = 0.001) and total cholesterol/HDL- cholesterol levels (P = 0.001), body mass index (P = 0.003) and frequency of hypertension (P = 0.001) were higher in patients than in control subjects.
Table 1. Demographic characteristics of the study population.
Frequencies of ABCA1 C69T polymorphism
Patient and control groups were in Hardy-Weinberg equilibrium for ABCA1 C69T genotypes (P = 0.310 and P = 0.440 respectively).
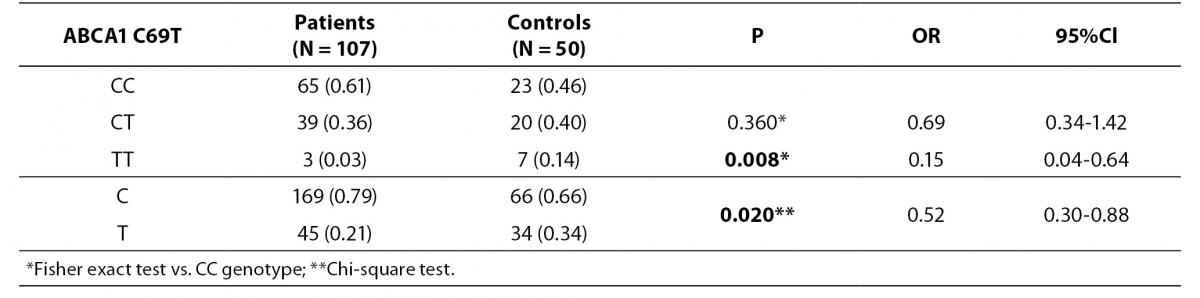
Distributions of C69T genotypes and alleles in study groups are shown in table 2. We have observed that the frequency of TT genotype is significantly higher in healthy controls compared to patients (14% vs. 3%; P = 0.008). Also frequency of T allele was higher in controls than in patients (34% vs. 21%; P = 0.020; OR (95% CI) = 0.52 (0.30-0.88)).
Table 2. Distribution of ABCA1 C69T genotypes and alleles in study groups.
Relationship between polymorphism and plasma lipid levels
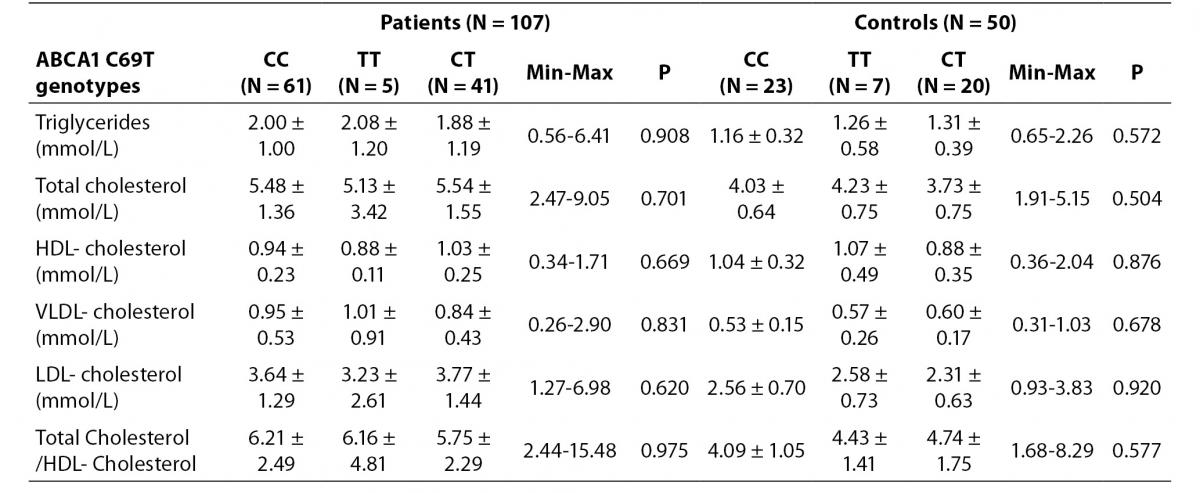
We could not find any relationship between ABCA1 C69T genotypes and lipid profiles. No association was found in HDL-cholesterol concentration according to the genotypes (Table 3).
Table 3. Distribution of lipid levels according to ABCA1 C69T genotypes in study groups.
Discussion
ABCA1 has an important role in the reverse transport of cholesterol from peripheral tissues to the liver via HDL-cholesterol. Mutations in ABCA1 gene may induce several lipid transport defects of HDL-cholesterol (18). Numerous of studies on the relationship between ABCA1 and diabetes mellitus have been done in recent times. Vergeer et al. showed that heterozygous carriers of ABCA1 mutations displayed mild hyperglycemia with no difference in insulin response after an oral glucose challenge compared with noncarriers of similar age, sex, and BMI (19). In another study, Patel et al. indicated that ABCA1 expression and protein concentrations in leukocytes, as well as function in cultured skin fibroblasts, are reduced in type 2 diabetic patients (20). Brunham et al. reported that a new role for ABCA1 in mediating cholesterol homeostasis and insulin secretion in pancreatic β-cells. According to their results, ABCA1 was highly expressed in β-cells, and absence of β-cell ABCA1 resulted in accumulation of cellular cholesterol, marked reduction in insulin secretion in vivo and a progressive impairment in glucose tolerance. This result gives us important information about ABCA1 genes and diabetes risk (5). Gao et al. showed that reduction in the concentration of HDL-C may be responsible for accelerated atherosclerosis in diabetic patients with poor glycemic control (10).
In previous studies, a lot of ABCA1 polymorphisms were investigated in lipid related diseases such as coronary artery disease and diabetes mellitus (21,22).
In a previously published study, recently found frequent non-synonymous R230C variant within ABCA1 gene was associated with HDL-cholesterol concentration, obesity and type 2 diabetics in Mexican Mestizos. It was determined that cells expressing the C230 allele showed a 27% cholesterol efflux reduction confirming that this variant has a functional effect in vitro. Also, C230 allele was associated with lower HDL-cholesterol concentration and higher body mass index (23). Harada et al. provided an evidence that the I/M 823 variant, not the R/K 219 variant, in the ABCA1 gene is a determinant of the HDL-cholesterol level. In addition, these authors showed the importance of this gene on lipid metabolism in Japanese patients with CAD (24). Tregouet et al. found that the ABCA1 R219K variant is associated with myocardial infarction risk (25).
Liu et al. suggested that the -191 G/C SNP in the promoter region of the ABCA1 gene is associated with CAD (26). Hong et al. found that the ABCA1 G2265 T variant may lead to decreased HDL-cholesterol (27). Porchay-Baldérelli et al. observed that the M allele of the I883M SNP was associated with higher HDL-cholesterol concentration and also the minor allele of the +378GNC SNP was associated with lower HDL-cholesterol concentration in 3129 French people (28). Saleehen et al. found a novel mutation in ABCA1 gene associated with low HDL-cholesterol concentration and type 2 diabetes mellitus (29).
In current study we have investigated ABCA1 C69T polymorphism in type 2 diabetic patients. According to the previous studies, our first aim was to explore possible relationship between ABCA1 C69T polymorphism and lipid concentrations in diabetic patients. However, we did not find any relationship between lipid levels and C69T gene polymorphism in both study groups. Whereas, in our previous study, we observed that the C69T polymorphism was associated with HDL-cholesterol concentration (30). In another Turkish study, Hodoglugil et al. evaluated polymorphisms in the ABCA1 gene in Turks, which is a population characterized by low HDL-cholesterol concentration. They observed that rare alleles of the C14T and V771M polymorphisms were associated with higher HDL-cholesterol concentration in men (31).
However, similarly to our results, some studies have reported no association between ABCA1 polymorphisms and lipid profile. Finnish Study suggested that the ABCA1 locus was not linked to HDL-cholesterol concentration in the coronary heart disease (32).
Nevertheless, we have found significantly higher frequency of both T allele and genotype in control group when compared to patients that made us think that T allele may be a protective factor against diabetes mellitus. But, we could not a relationship between genotypes and lipid concentrations in our two groups.
The present study has some potential limitations, the small number of study groups being the most important one. This could be a reason for some of the results that demonstrated no statistical significance. Studies with a larger sample size in different races will help us to understand relationship between ABCA1 C69T polymorphism and lipid parameters in diabetes mellitus.
Notes
Potential conflict of interest
None declared.
References
1. Mooradian AD. Dyslipidemia in type 2 diabetes mellitus. Nat Clin Pract Endocrinol Metab 2009;5:150-9.
2. Beckman JA, Creager MA, Libby P. Diabetes and Atherosclerosis: epidemiology, pathophysiology and management. JAMA 2002;287:2570-81.
3. Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97:1837-47.
4. Mazzone T, Chait A, Plutzky J. Cardiovascular disease risk in type 2 diabetes mellitus: insights from mechanistic studies. Lancet 2008;371:1800-9.
5. Brunham LR, Kruit JK, Pape TD, Timmins JM, Reuwer AQ, Vasanji Z, et al. β-Cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment. Nat Med 2007;13:340-7.
6. Albrecht C, Soumian S, Amey JS, Sardini A, Higgins CF, Davies AH, et al. ABCA1 expression in carotid atherosclerotic plaques. Stroke 2004;35:2801-6.
7. Porchay I, Pean F, Belili N, Royer B, Cogneau J, Chesnier MC, et al. ABCA1 single nucleotide polymorphisms on high-density lipoprotein cholesterol and overweight: the D.E.S.I.R. Study. Obesity 2006;14:1874-9.
8. Villarreal-Molina MT, Aguilar-Salinas CA, Rodríguez-Cruz M, Riaño D, Villalobos-Comparan M, Coral-Vazquez R, et al. The ATP-binding cassette transporter A1 R230C variant affects HDL cholesterol levels and BMI in the Mexican population: association with obesity and obesity-related comorbidities. Diabetes 2007;56:1881-87.
9. Salinas CA, Cruz-Bautista I, Mehta R, Villarreal-Molina MT, Pérez FJ, Tusié-Luna MT, Canizales-Quinteros S. The ATP-binding cassette transporter subfamily A member 1 (ABC-A1) and type 2 diabetes: an association beyond HDL cholesterol. Curr Diabetes Rev 2007;3:264-7.
10. Gao F, Yan T, Zhao Y, Yin F, Hu C. A possible mechanism linking hyperglycemia and reduced high-density lipoprotein cholesterol levels in diabetes. J Huazhong Univ Sci Technolog Med Sci 2010;30:318-21.
11. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabets Care 2004;27:S5-10.
12. World Health Organisation. Physical status: the use an interpretation of anthropometry: report of a WHO expert committee. WHO Tech Rep Ser 1995;854:1-452.
13. Friedewald WT, Levy RI, Fredrickson DS. Estimation of low density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin Chem 1972;18:499-508.
14. Dimeski G, Jones BW. Lipaemic samples: Effective process for lipid reduction using high speed centrifugation compared with ultracentrifugation. Biochem Med 2011;21:86-94.
15. Miller SA, Dykes DD, Polesky HS. Simples salting out procedure for extracting DNA from human nucleated cells. Nucleic Acid Res 1988;16:1215.
16. Zwarts KY, Clee SM, Zwinderman AH, Engert JC, Singaraja R, Loubser O, et al. ABCA1 regulatory variants influence coronary artery disease independent of effects on plasma lipid levels. Clin Genet 2002;61:115-25.
17. Marusteri M, Bacarea V. Comparing groups for statistical differences: how to choose the right statistical test? Biochem Med 2010;20:15-32.
18. Oram JF. Tangier disease and ABCA1. Biochim Biophys Acta 2000;1529:321-30.
19. Vergeer M, Brunham LR, Koetsveld J, Kruit JK, Verchere CB, Kastelein JJ, et al. Carriers of loss-of-function mutations in ABCA1 display pancreatic beta-cell dysfunction. Diabetes Care 2010;33:869-74.
20. Patel DC, Albrecht C, Pavitt D, Paul V, Pourreyron C, Newman SP, et al. Type 2 diabetes is associated with reduced ATP-binding cassette transporter A1 gene expression, protein and function. PLoS One 2011;6:e22142.
21. Stefkova J, Poledne R, Hubacek JA. Polymorphisms in ABCA1 transporter and plasma lipids. Physiol Res 2004;53:235-43.
22. Sheĭdina AM, Pchelina SN, Demidova DV, Rodygina TI, Taraskina AE, Toperverg OB, et al. Allele frequency analysis of four single nucleotide polymorphisms locating in promoter and 5’- untranslated regions of ABCAI gene in young men – survivors from myocardial infarction. Kardiologiia 2004;44:40-5.
23. Acuña-Alonzo V, Flores-Dorantes T, Kruit JK, Villarreal-Molina T, Arellano-Campos O, Hünemeier T, et al. A functional ABCA1 gene variant is associated with low HDL-cholesterol levels and shows evidence of positive selection in Native Americans. Hum Mol Genet 2010;19:2877-85.
24. Harada T, Imai Y, Nojiri T, Morita H, Hayashi D, Maemura K, et al. A common Ile 823 Met variant of ATP-binding cassette transporter A1 gene (ABCA1) alters high density lipoprotein cholesterol level in Japanese population. Atherosclerosis 2003;169:105–12.
25. Tregouet DA, Ricard S, Nicaud V, Arnould I, Soubigou S, Rosier M, et al. In-depth haplotype analysis of ABCA1 gene polymorphisms in relation to plasma ApoA1 levels and myocardial infarction. Arterioscler Thromb Vasc Biol 2004;24:775-81.
26. Liu L, Guo ZG, Wang QG, Liu SL, Lai WY, Tu Y. Significance of -191G/C single nucleotide polymorphisms in the promoter region of ATP binding cassette transporter gene in coronary artery disease. Di Yi Jun Yi Da Xue Xue Bao 2005;25:660–2.
27. Hong SH, Riley W, Rhyne J, Friel G, Miller M. Lack of association between increased carotid intima-media thickening and decreased HDL-cholesterol in a family with a novel ABCA1 variant, G2265T. Clin Chem 2002;48:2066-70.
28. Porchay-Baldérelli I, Péan F, Emery N, Maimaitiming S, Bellili N, Travert F, et al. Relationships between common polymorphisms of adenosine triphosphate-binding cassette transporter A1 and high-density lipoprotein cholesterol and coronary heart disease in a population with type 2 diabetes mellitus. Metabolism 2009;58:74-9.
29. Saleheen D, Nazir A, Khanum S, Nazir A, Ahmad U, Khalid H, et al. R1615P: a novel mutation in ABCA1 associated with low levels of HDL and type II diabetes mellitus. Int J Cardiol 2006;110:259-60.
30. Ergen A, Isbir S, Tekeli A, Isbir T. Investigation of ABCA1 C69T and G191C polymorphisms in coronary artery disease. In Vivo 2008;22:187-90.
31. Hodoglugil U, Williamson DW, Huaung Y, Mahley RW. Common polymorphisms of ATP binding cassette transporter A1, including a functional promoter polymorphism, associated with plasma high density lipoprotein levels in Turks. Atherosclerosis 2005;183:199-212.
32. Kakko S, Kelloniemi J, von Rohr P, Hoeschele I, Tamminen M, Brousseau ME, et al. ATP-binding cassette transporter A1 locus is not a major determinant of HDL-C levels in a population at high risk for coronary heart disease. Atherosclerosis 2003;166:285-90.