Incorrect order of draw could be mitigate the patient safety: a phlebotomy management case report
Gabriel Lima-Oliveira
[*]
[1]
Giuseppe Lippi
[2]
Gian Luca Salvagno
[3]
Martina Montagnana
[3]
Geraldo Picheth
[4]
Gian Cesare Guidi
[5]
[1] Laboratory of Clinical Biochemistry, Department of Life and Reproduction Sciences, University of Verona, Verona, Italy, Post-Graduate Program of Pharmaceutical Sciences, Department of Medical Pathology Federal University of Parana, Curitiba, Parana, Brazil, MERCOSUL: Sector Committee of Clinical Analyses and in Vitro Diagnostics – CSM 20, Rio de Janeiro, Brazil, Brazilian Society of Clinical Analyses on Sao Paulo State, Brazil
[2] Clinical Chemistry and Hematology Laboratory, Department of Pathology and Laboratory Medicine, Academic Hospital of Parma, Parma, Italy
[3] Laboratory of Clinical Biochemistry, Department of Life and Reproduction Sciences, University of Verona, Verona, Italy
[4] Post-Graduate Program of Pharmaceutical Sciences, Department of Medical Pathology Federal University of Parana, Curitiba, Parana, Brazil
[5] Laboratory of Clinical Biochemistry, Department of Life and Reproduction Sciences, University of Verona, Verona, Italy, Post-Graduate Program of Pharmaceutical Sciences, Department of Medical Pathology Federal University of Parana, Curitiba, Parana, Brazil
Introduction
The preanalytical phase (PP) is nowadays recognized as the most vulnerable part of the total testing process (1). The PP procedures involving phlebotomy are critical for obtaining diagnostic blood specimens: on the whole they represent a well known and recognized problem, probably among the most important issues in laboratory medicine (2-5). Lippi and Simundic recently advised to think outside the box and to address the extra-analytical processes that are more vulnerable to errors (6). With this aim, we demonstrate here that incorrect procedures during phlebotomy can be a source of spurious hyperkalaemia and hypocalcemia in patients’ samples.
Case report
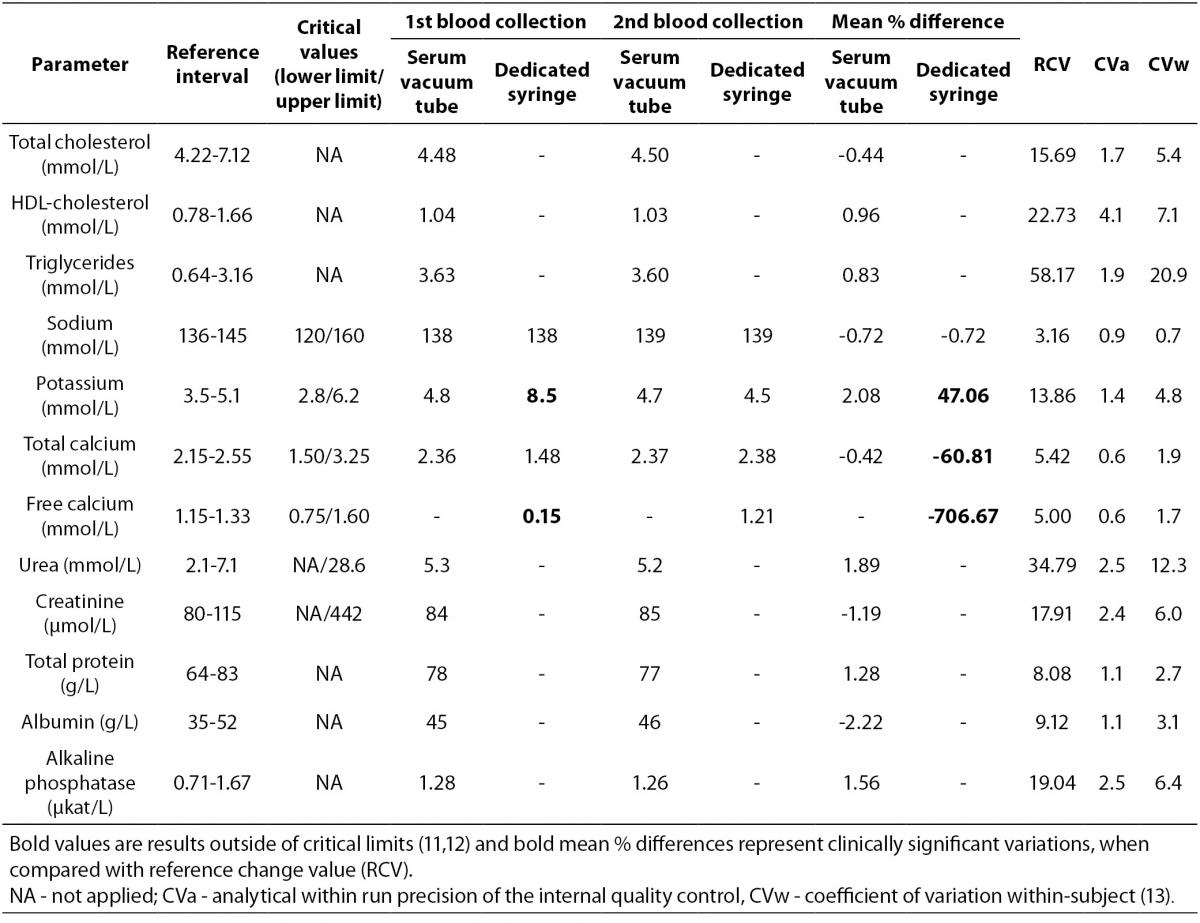
A 45 years old male outpatient, with no apparent clinical complaints, wheelchair dependent, calls to our laboratory accredited by DICQ® (DICQ is a National System of Accreditation from Brazilian Society of Clinical Analyses based on ISO 15189 document), asking for a blood specimen collection at home, for performing a series of routine laboratory tests prescribed by his physician. The tests are the: total cholesterol (CHOL), HDL-cholesterol (HDL), triglycerides (TG), sodium (Na), potassium (K), total calcium (tCa), free calcium (fCa), urea, creatinine (CREA), total protein (TP), albumin (ALB), alkaline phosphatase (ALP) and routine blood count with platelets (CBC). The patient was asked to observe 12-hours overnight fasting and the blood collection was scheduled for 7 am, 3 work days after his call. Before blood collection, the median cubital vein was located on the left forearm using only a subcutaneous tissue transilluminator device (Venoscópio IV plus, Duan do Brasil, Sao Paulo, Brazil) –without tourniquet – to prevent interference from venous stasis (7-9), and blood samples were collected using a 20-G straight needle (BD Vacuntainer ® Becton, Dickinson and Company Franklin Lakes, NJ, USA) connected to the holder, directly into the vacuum tubes (Greiner Bio-One GmbH, Kremsmünster, Austria). The order of different types of tube filling was: a) clot activator and gel separator tube, b) K3EDTA tube for CBC and c) a blood gas dedicated-syringe (Pico 50® with 80 I.U. lyophilized electrolyte-balanced lithium heparin, Radiometer Medical ApS, Denmark) without needle, which was directly connected to the needle-holder system in order to avoid double vein puncture. After collection, the tubes were properly mixed and transported from patient’s house to the core laboratory by car at 20 ± 2 °C in upright position inside a transport box. The blood into syringe was transported at 4 ± 1 °C, as recommended by clinical laboratory standard institute C31-A2 document (10). The blood specimens (serum tube, cell blood count tube and dedicated syringe) arrived in core laboratory about one hour after blood collection and were immediately processed and analyzed (< 15 min from the arrival time). The dedicated syringe was properly mixed and the free calcium was measured by ion-selective electrode on ABL 700® (Radiometer Medical ApS, Brønshøj, Denmark), according to the manufacturer’s specifications and using proprietary reagents. Whole blood for CBC was gently mixed and analyzed on Sysmex® XE-2100D, Automated Hematology Analyzer (Sysmex Corporation®, Kobe, Japan). Serum vacuum tube was centrifuged at 1500 x g for 10min at room temperature. In sequence, the laboratory tests on serum were performed on the primary blood tube on Cobas® 6000 < c501 > module (Roche Diagnostics GmbH, Penzberg, Germany), according to the manufacturer’s specifications and using proprietary reagents. All laboratory instruments had been previously calibrated against appropriate proprietary reference standard material and verified with the use of proprietary controls. Our evaluation of the within-run precision by internal quality control is shown as coefficients of variation (CVa). The results are shown in table 1. The laboratory staff (clinical chemistry specialist) contacted the patient by phone because some results (i.e. potassium, total calcium and free calcium) of the sample collected in dedicated syringe were out of the critical limits established by Kost (11,12). The patient reported that he did not use topical medicines or intravenous solution in the last four months. A new blood collection (serum tube and dedicated syringe only) was scheduled for 12.30 pm of the same day. The venipuncture for the 2nd blood collection was performed, by the same phlebotomist, in cephalic vein on the left forearm. The drawing order was: a) clot activator and gel separator tube, b) blood gas dedicated-syringe; both of the same brand and lot used for the first blood collection. So, during the 2ndvenipuncture the diagnostic blood specimen collection did not include a new EDTA vacuum tube. The following procedures were identical to the described above for the 1st blood specimen collection. The 2nd blood specimens arrived in core laboratory about 45 min after collection and were immediately processed and analyzed on the same analytical instruments. The results are shown in table 1.
Table 1. Laboratory results
Statistical analysis
The reference change values (RCV) were determined to ascertain any clinically significant differences between the first and the second blood collection according to the formula RCV = 21/2 x Z x [(CVa)2 + (CVw)2]1/2 where Z: z-score is 1.96 (5% standard normal deviation in bicaudal testing); CVa: analytical within run precision of the internal quality control and CVw: coefficient of variation within-subject (13) were applied. Mean % differences were determined according to the formula difference % mean = [(1st blood collection – 2nd blood collection)/1st blood collection] x 100.
Discussion
In clinical laboratory the quality indicators are fundamental tools for quantifying the quality of a selected aspect of care by comparing it against a defined goal. For private laboratories the level of patient satisfaction with the diagnostic blood collection service is a very important quality indicator. With the aim to increase the patient satisfaction the procedure described above was implemented approximately six months ago in our laboratory, in order to avoid double venipuncture. This procedure is not explicitly stated in both international and national guidelines but is frequently performed in laboratories of South America countries. In order to avoid possible test result errors due to additive carryover, the correct order of blood drawing by venipuncture should be as follows: i) blood culture tube or tube without additive; ii) sodium citrate tube; iii) serum tube with or without clot activator and with or without gel separator; iv) heparin tube with or without gel separator and/or dedicated-syringe with heparin; v) EDTA tube with or without gel separator; and vi) glycolytic inhibitor tube. The Clinical Laboratory Standard Institute H03-A6 document (14) has standardized order of draw based on Calam and Cooper results (15). This document is widely used by laboratory quality managers to standardize the blood collection procedures (16). When our laboratory employees were retrained to eliminate this non conformity, regarding draw order, they reported that: “During the maneuver to insert the syringe into the adapter of the tube holder, sometimes we risk to miss the vein: for this reason and thus to avoid repeating venipunctures, we would like to insert the syringe at the end of all tubes rather than at mid blood collection.”Nonetheless, this patient’s case demonstrates that something wrong can still arise from an apparently standardized procedure of tube order for blood drawing. At first sight, when looking at the potassium and calcium critical values from the 1stcollected patient’s sample (dedicated-syringe), a main hypothesis of such a severe modification of blood potassium and calcium could be a chronic kidney disease. Obviously this supposition was discarded after checking Urea, CREA, TP and ALB results from the 1st and 2nd blood collections, thus the condition of the patient was judged far from critical. Until now two studies agreed in showing that the order of tube drawing did not affect routine biochemistry (e.g. potassium, magnesium, alkaline phosphatase, and iron), when closed system for blood collection from Sarstedt or BD were used (17,18). Unfortunately these studies did not measure the calcium values in serum tubes collected after EDTA vacuum tubes and/or vacuum tubes produced by Greiner Bio-One. Moreover we previously found that different brands of in vitrodiagnostic devices (e.g. vacuum tubes and syringes) have different performances in routine laboratory tests and that changes in the brands can increase laboratory variability (19-23). Future investigations should be planned to cover the impact of vacuum tubes brands on EDTA carryover. Several others articles showed spurious hyperkalaemia and hypocalcemia based on EDTA carryover (24-26). Cornes et al. after measuring EDTA in 117 hyperkalaemic samples showed that spurious hyperkalaemia due to kEDTA contamination was common (about 25% of all samples tested). In the 27 patients retested, serum potassium was within the reference range confirming the contamination with EDTA (24).In the present case we have estimated an EDTA carryover of 0.5 ~ 0.6 mg/mL of blood into the dedicated syringe from the preceding EDTA-containing tube. It was predicted by stoichiometric calculation based on the observed reduction of Ca ions and on the molecular mass of EDTA. The estimation is based on the variation of the potassium concentration, from the K3EDTA tube to the syringe, taking into account that a mass concentration of 1.5 mg of K3EDTA per 1 mL of blood as an optimal anticoagulant level is declared (27). The K3EDTA carryover is the best hypothesis to justify the spurious results found in the dedicated syringe from the 1st blood collection. Moreover when looking at total- and free-calcium results the decreased results observed in dedicated syringe appear not so proportionally reduced. Theoretically, the total amount of calcium in plasma is about 2.5 mmol/L and the normal amount of calcium in plasma that can be complexed by a sequestering agent is 1.15 mmol/L. The remaining calcium is bound to protein (e.g. albumin) and is scarcely available for sequestration by EDTA (27). In any case clinically significant differences were observed for potassium, total calcium and free calcium only in the blood of the dedicated syringe when mean percent differences were compared with RCV derived from biological variation (Table 1) (28). Obviously the quality specifications derived from biological variation are considered both very important and useful in the daily practice by the quality managers of the medical laboratories (13,29,30). Based on this depictive case we recommend the laboratory managers to train the phlebotomists as follows: 1) the dedicated needleless syringes for blood-gas must be connected to the needle-holder system immediately after serum tubes and before EDTA vacuum tubes; 2) to avoid double vein puncture the dedicated needleless syringes for free calcium determination must be connected to the needle-holder system immediately after serum tubes and before EDTA vacuum tubes.
In conclusion, we caution the laboratory managers about the current procedures advising to connect the syringes for blood gas and calcium tests at the end of all vacuum tubes; moreover we claim that the continuous training of the phlebotomists and of all the laboratory staff with regards to the effects of preanalytical interferences is a mandatory task in every modern clinical laboratory.
Notes
Potential conflict of interest
None declared.
References
2. Bilic-Zulle L, Simundic AM, Supak-Smolcic V, Nikolac N, Honovic L. Self reported routines and procedures for the extra-analytical phase of laboratory practice in Croatia -cross-sectional survey study. Biochem Med 2010;20:64-74.
http://dx.doi.org/10.11613/BM.2010.008.
3. Lippi G, Salvagno GL, Montagnana M, Guidi GC. The skilled phlebotomist. Arch Pathol Lab Med 2006;130:1260-1.
4. Lippi G, Salvagno GL, Montagnana M, Lima-Oliveira G, Guidi GC, Favaloro EJ. Quality Standards for Sample Collection in Coagulation Testing. Semin Thromb Hemost 2012;38:565-75.
http://dx.doi.org/10.1055/s-0032-1315961. 5. Lima-Oliveira G, Guidi GC, Salvagno GL, Montagnana M, Rego FGM, Lippi G, et al. Is phlebotomy part of the dark side in the clinical laboratory struggle for quality? Lab Med 2012;43:17-21.
http://dx.doi.org/10.1309/LMZ7YARD6ZSDIID.
7. Lima-Oliveira G, Lippi G, Salvagno GL, Montagnana M, Mangueira C, Sumita N, et al. New ways to deal with known preanalytical issues: use of transilluminator instead of tourniquet for easing vein access and eliminating stasis on clinical biochemistry. Biochem Med 2011;21:152-9.
http://dx.doi.org/10.11613/BM.2011.024.
8. Lima-Oliveira G, Lippi G, Salvagno GL, Montagnana M, Scartezini M, Guidi GC, et al. Transillumination: a new tool to eliminate the impact of venous stasis during the procedure for the collection of diagnostic blood specimens for routine haematological testing. Int J Lab Hematol 2011;33:457-62.
9. Lima-Oliveira G, Salvagno GL, Lippi G, Montagnana M, Scartezini M, Picheth G, et al. Elimination of the venous stasis error for routine coagulation testing by transillumination. Clin Chim Acta 2011;412:1482-4.
http://dx.doi.org/10.1016/j.cca.2011.04.008.
10. Clinical Laboratory Standards Institute. Calcium determinations: precollection variables, specimen choice, collection, and handling. CLSI C31-A2 document. 2nd ed. Wayne, PA: Clinical Laboratory Standards Institute; 2001.
11. Kost GJ. The significance of ionized calcium in cardiac and critical care. Availability and critical limits at US medical centers and chidren’s hospitals. Arch Pathol Lab Med 1993;117:890-6.
12. Kost GJ. Using critical limits to improve patient outcome. MLO Med Lab Obs 1993;25:22-7.
14. Clinical Laboratory Standards Institute. Procedures for the collection of diagnostic blood specimens by venipuncture. CLSI H3-A6 document. 6th ed. Wayne, PA: Clinical Laboratory Standards Institute; 2007.
15. Calam RR, Cooper MH. Recommended “order of draw” for collecting blood specimens into additive-containing tubes. Clin Chem 1982;28:1399.
16. Lima-Oliveira G, Lippi G, Salvagno GL, Montagnana M, Picheth G, Guidi GC. Impact of the phlebotomy training based on CLSI/NCCLS H03-A6 - procedures for the collection of diagnostic blood specimens by venipuncture. Biochem Med 2012;22:342-51.
http://dx.doi.org/10.11613/BM.2012.036.
17. Sulaiman RA, Cornes MP, Whitehead SJ, Othonos N, Ford C, Gama R. Effect of order of draw of blood samples during phlebotomy on routine biochemistry results. J Clin Pathol 2011;64:1019-20.
http://dx.doi.org/10.1136/jclinpath-2011-200206.
18. Cornes MP, Sulaiman RA, Whitehead SJ, Othonos N, Ford C, Gama R. Incorrect order of draw of blood samples does not causes potassium EDTA sample contamination. Brit J Biomed Sci 2012;69:1-2.
19. Lima-Oliveira G, Lippi G, Salvagno GL, Montagnana M, Picheth G, Guidi GC. Different manufacturers of syringes: A new source of variability in blood gas, acid-base balance and related laboratory test? Clin Biochem 2012;45:683-7.
http://dx.doi.org/10.1016/j.clinbiochem.2012.03.007.
20. Lima-Oliveira G, Lippi G, Salvagno GL, Montagnana M, Poli G, Solero GP, et al. K3EDTA vacuum tubes validation for routine hematological testing. ISRN Hematology 2012;2012:875357.
21. Lima-Oliveira G, Lippi G, Salvagno GL, Montagnana M, Picheth G, Guidi GC. Pre analytical management: serum vacuum tubes validation for routine clinical chemistry. Biochem Med 2012;22:180-6.
http://dx.doi.org/10.11613/BM.2012.021.
22. Lima-Oliveira G, Lippi G, Salvagno GL, Montagnana M, Picheth G, Guidi GC. Sodium citrate vacuum tubes validation: preventing preanalytical variability in routine coagulation testing. Blood Coagul Fibrinolysis 2013;24:252-5.
http://dx.doi.org/10.1097/MBC.0b013e32835b72ea.
23. Lima-Oliveira G, Lippi G, Salvagno GL, Montagnana M, Poli G, Solero GP, et al. The brand of K2EDTA vacuum tubes as a new source of preanalytical variability in routine hematology testing. Brit J Biomed Sci 2013;70:6-9.
26. Naguib MT, Evans N. Combined false hyperkalemia and hypocalcemia due to specimen contamination during routine phlebotomy. South Med J 2002;95:1218-20.
27. National Committee for Clinical Laboratory Standards. Tubes and additives for venous blood specimen collection. NCCLS document H1-A5. 5th ed. Wayne, PA: National Committee for Clinical Laboratory Standards; 2003.
28. Ricos C, Alvarez V, Cava F, Garcia-Lario JV, Hernandez A, Jimenez CV, et al. Current databases on biological variation: pros, cons and progress. Scand J Clin Lab Invest 1999;59:491-500.
http://dx.doi.org/10.1080/00365519950185229.
29. Cembrowski GS, Tran DV, Higgins TN. The use of serial patient blood gas, electrolyte and glucose results to derive biologic variation: a new tool to assess the acceptability of intensive care unit testing. Clin Chem Lab Med 2010;48:1447-54.
http://dx.doi.org/10.1515/cclm.2010.286.
30. Ricos C, Cava F, Garcia-Lario JV, Hernandez A, Iglesias N, Jimenez CV, et al. The reference change value: a proposal to interpret laboratory reports in serial testing based on biological variation. Scand J Clin Lab Invest 2004;64:175-84.
http://dx.doi.org/10.1080/00365510410004885.