Introduction
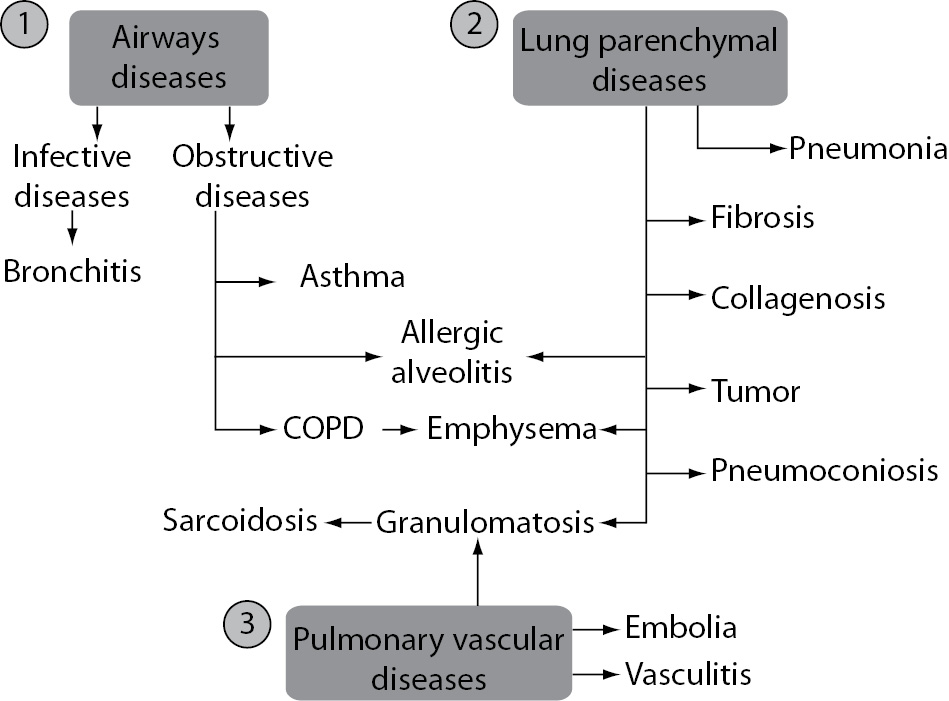
The main function of the lungs is respiratory function, i.e. respiratory gas exchange. In addition, the lungs have other important non-respiratory functions. The latter include the maintenance of a stable acid-base balance, immunological functions and metabolic activities. Respiratory diseases are the most common diseases in children and adolescents (e.g. allergic diseases, infections of respiratory system) and adults (e.g. chronic obstructive pulmonary disease, COPD). Generally, respiratory diseases include three main groups: airways diseases, lung parenchymal diseases and pulmonary vascular diseases (Figure 1).
Figure 1. Respiratory diseases: airways diseases (1), lung parenchymal diseases (2) and pulmonary vascular diseases (3). COPD - chronic obstructive pulmonary disease.
In some diseases, there is overlap between pathological hallmarks, e.g. allergic alveolitis and emphysema are airways and lung parenchymal diseases; granulomatosis is both, lung parenchymal and pulmonary vascular disease. In industrialized countries, the morbidity of infectious diseases are decreasing, and morbidity of obstructive diseases, particularly asthma and chronic obstructive pulmonary disease (COPD) have been increasing in the past century (1).
The diagnosis of respiratory diseases and the evaluation of therapeutic outcome are based on history data, clinical symptoms, pulmonary function testing and imaging techniques. In addition, for direct assessment of inflammation invasive procedures to obtain bronchoalveolar lavage (BAL), biopsy specimens, induced sputum are used in everyday practice. However, these procedures are used when strongly indicated, especially in young children (2,3). Therefore, over the past three decades, the goal of many researchers has been to approve analysis of breath and exhaled breath condensate (EBC) as noninvasively obtained samples (2,3).
More than 30 years ago, Sidorenko et al. in the former Soviet Union started with the analysis of EBC, and the first article was published in Russian language in 1980 (4). Over the next decade, only Russian scientists have supported the idea that EBC may be a promising matrix for the diagnosis of lung diseases. From 1992, after Western researches published their first EBC paper (5), the number of published articles has been increasing progressively. During the period from 1980 to 2012, databases PubMed, OVID and Scopus have so far included 869, 917 and 3628 published articles on EBC, respectively. The following authors have investigated EBC systematically and have published more than 10 articles in the field: Montuschi P. (76 articles), Barnes PJ. (60 articles), Corradi M. (49 articles), Kharitonov SA. (39 articles), Carpagnano GE. (38 articles), Horvath I. (34 articles), Mutti A. (27 articles), Hunt J. (23 articles), Antus B. (16 articles), Antczak A. (14 articles), Effros RM. (13 articles), Kostikas K. (13 articles), Rosias PP. (11 articles) and their colleagues. In Croatia, two groups of researchers made a modest contribution to the investigation of EBC analysis: authors from Srebrnjak Children Hospital, Zagreb (Dodig S, et al., analysis of EBC in children, 9 articles) and authors from Institute for Medical Research and Occupational Health, Zagreb (Ljubičić Ćalušić A, et al., analysis of EBC in adults, 3 articles). Many papers pay attention to the problems in EBC determination (both, clinical and analytical problems). If analytical problems are not solved, conclusions based on clinical investigations will be uncertain. Therefore, the aim of the present review was to accentuate some analytical problems in EBC analysis.
Clinical laboratory specialists should assume responsibility for the total analytical process (total testing cycle) in laboratory medicine, from the patient preparation, test selection, leading to sample collection, transport to the laboratory, sample handling and storage, sample analysis and finally, results report. These activities are classified into three phases - preanalytical, analytical and postanalytical. This paper gives a review of some solved and unsolved problems typical for the total testing cycle of EBC.
Preanalytical phase of EBC analysis
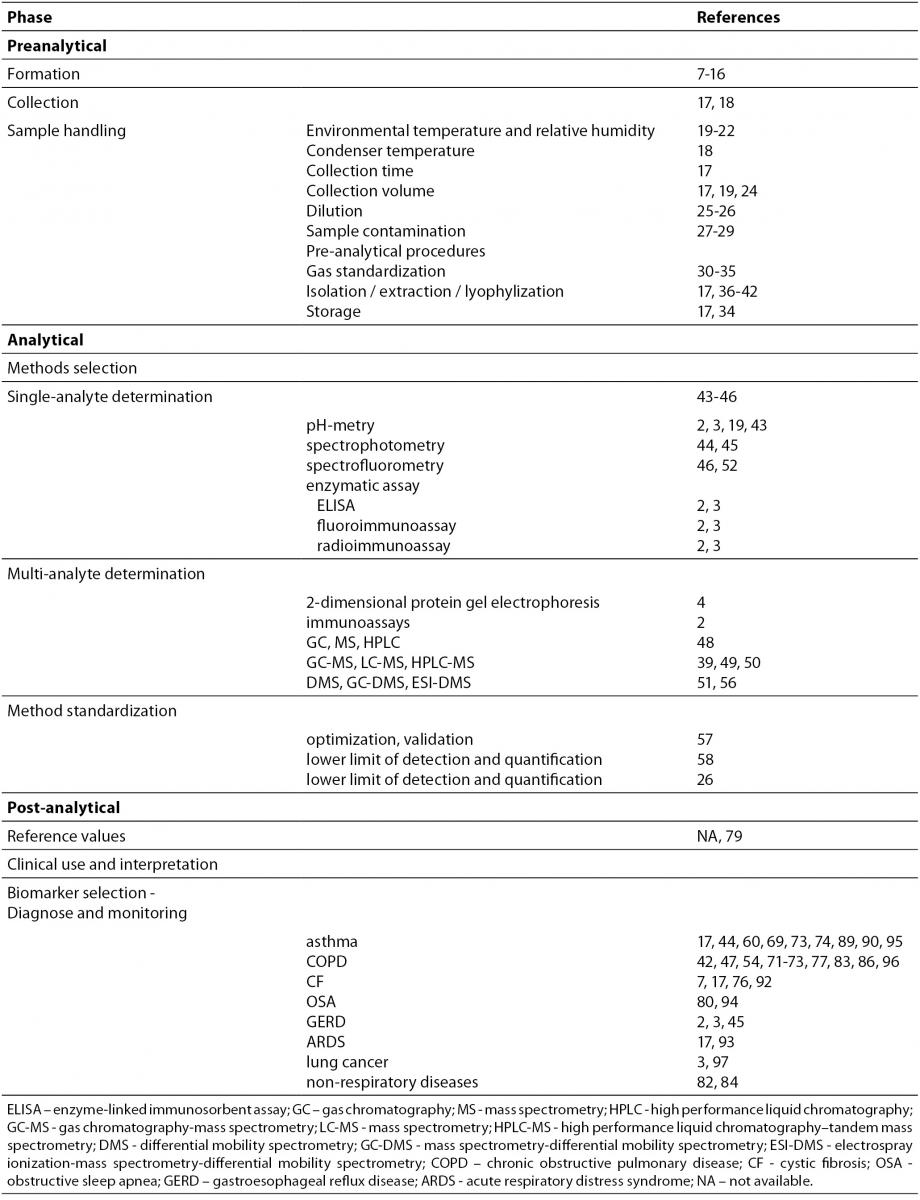
Laboratory professionals are aware that preanalytical phase in laboratory medicine is a very important component of laboratory testing (6,7). In the last two decades, a great attention was paid to define preanalytical issues in EBC analysis – from formation, sampling, sample handling, environmental factors, and dilution to appropriate handling and storage (Table 1).
Table 1. Pre-analytical, analytical and post-analytical phases of exhaled breath condenste analysis.
Formation of EBC
Exhaled breath contains thousands of volatile (such as oxygen, carbon dioxide, nitric oxide, ethane, pentane) and non-volatile compounds, water vapour as well as liquid particles (i.e. droplets) and bioaerosols (bacteria, viruses, fungi) that derive from the respiratory tract (8,9). Droplets of respiratory tract lining fluid (RTLF) are released from the surfaces of the airways (Figure 2A). In addition, a great quantity of water is released as vapour (evaporation). During turbulence in the airways, i.e. during reopening of closed bronchioles and alveoli, non-volatile compound from epithelial lining fluid undergoes aerosolization (3,10). The droplet formation depends on the velocity of the flow of exhaled air and the surface tension (higher velocity/lower surface tension). The number of particles in exhaled air is very low, ranging from 0.1 - 4 particles x cm-3 (diameter < 0.3 μm) (11).
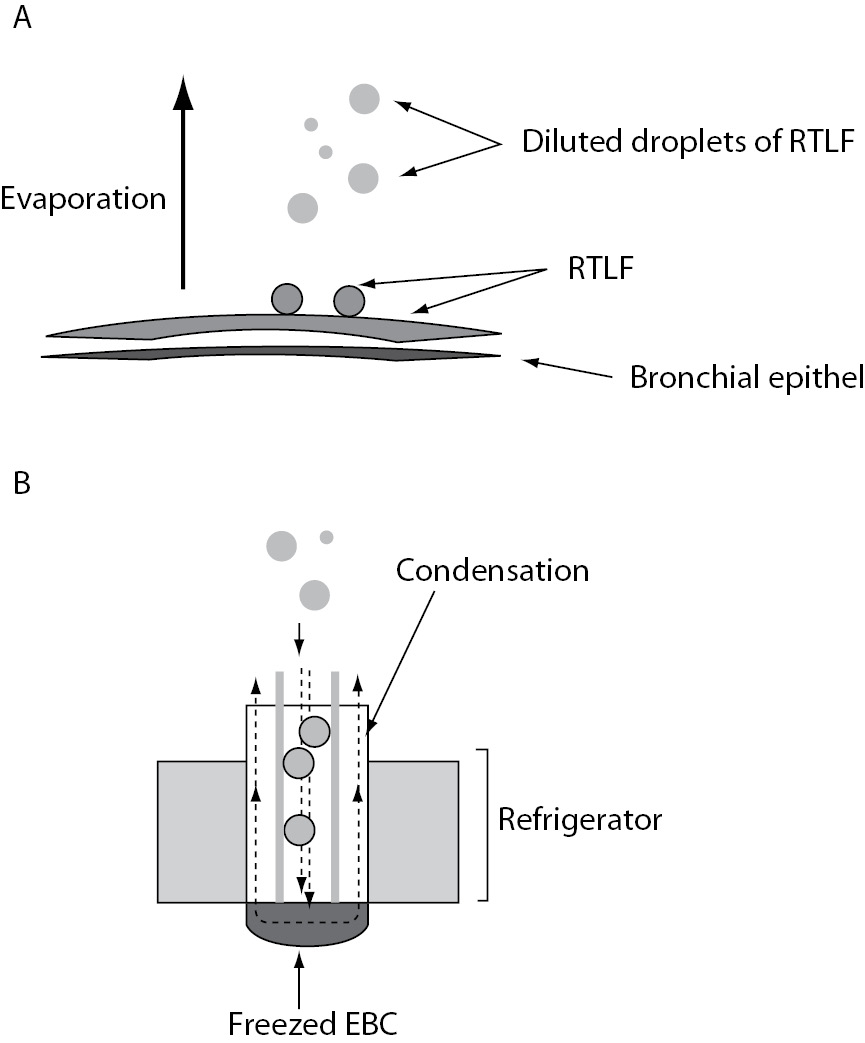
Figure 2. Formation of exhaled breath (A) and sampling of EBC in the cooled container (B): A – Droplets of respiratory tract lining fluid (RTLF) are released from the surfaces of the airways. A great quantity of water are released as vapor (evaporation); B – When droplets of RTLF reach the condenser, they become diluted by water vapor that are deposited on the walls of the refrigerated container (condenser).
RTLF is a thin liquid film that lines the respiratory epithelium. Its composition varies between conducting zone (airways) and the respiratory zone (respiratory bronchioles, alveolar ducts, and alveoli) (12). RTLF in the conducting zone consists of two phases: sol phase (watery) and gel phase (mucus). The sol phase contains soluble components of bronchial secretion and serum proteins, while gel phase contains bronchial glycoproteins, protein bound to the bronchial glycoproteins and some serum proteins (13). RTLF in the respiratory zone (surfactant) contains phospholipids (80%), cholesterol (10%), proteins (10%), and very small amounts of carbohydrates (12). EBC composition corresponds to the composition of RTLF, but in significantly lower concentrations (14). According to Effros et al., EBC consists of water vapour, and contains aerosol particles originating from the RTLF from the lower airways (15). However, according to Bondesson et al. (16), EBC derives mainly from the central airways, and its content may not correspond to the content of RTLF originating from smaller airways. Therefore, the exact anatomical site of origin and the precise mechanism of EBC formation are still a matter of dispute.
Collection of EBC
Optimized collectors for EBC sampling are available from various manufacturers, all based on the principle of “freezing exhaled breath” (Figure 2B). According to the American Thoracic Society (ATS)/European Respiratory Society (ERS) Task Forceonrecommendationsfor EBC (17) collectors should have an inert material on the condensing surface, e.g. glass, silicone, teflon, aluminium, polypropylene. There is no ideal device for collecting EBC for all biomarkers, since the concentrations of some biomarkers depend on adhesive properties of condenser coatings. The subject breathes calmly (tidal breath, respiratory rate 15-20 breaths/minute) via a mouth-piece (with nasal clip) connected to a tube that separates inhaled and exhaled air. During exhalation, the air passes through a condenser cooled to a low temperature (-5 oC to -30 oC for different manufacturers; usually at -20 oC), and EBC is collected in the cooled container (e.g. electric refrigerator) (3). Condensing temperature affects significantly the pH-values of EBC (pH is more acidic at condensing temperature -70 oC, than at -20 oC) (18). Therefore, the condensation temperature has to be reported to facilitate comparison of results obtained by different laboratories (3). In addition, levels of some biomarkers (e.g. oxidative stress markers) have shown circadian variations. Hence, the time of EBC collection should be recorded. Determination of biomarkers known to be affected by certain food, drinks or medications it is recommended to abstain from these a few hours before EBC collection (Figure 2) (3,17).
Influence of environmental temperature and relative humidity
Environmental conditions, i.e. relative humidity (RH) and temperature may influence on the volume of EBC and on the determination of some constituents in EBC sample (pH-values). Smaller volume may be obtained at lower RH (19). RH of inspired air influences the rate at which EBC is formed (20) and the diameter of an expired droplet (21). The diameter of exhaled particles is smaller and supersaturated at lower ambient RH. Ambient temperature affects significantly the pH-values of EBC. pH is more acidic at 23 oC than at 37 oC (22). Therefore ambient temperature RH have to be under control in the room, where the EBC is collected.
Collection time of EBC
Ventilatory rate is the major determinant of the amount of EBC over time. With respiratory rate of 15-20 breaths/minute, sufficient sample may be collected during 10-15 minutes (i.e. 150–300
breaths).
Volume of EBC
A strong positive correlation was found between EBC volume and total respired volume and tidal volume, respectively (23). Volume of collected EBC does not depend on lung function parameters, e.g. forces expiratory volume in 1 second, (FEV1). As the amount of EBC increases significantly with higher minute ventilation (19), the volume of EBC varies among individuals. According to Lema et al. (24) the relation between the volume of inhaled air and the volume of collected EBC for each subject is expressed by the equation:
volume of EBC (mL) = 0.013 × volume of inhaled air (L) + 0.255 (24).
If respiratory rate remains constant during EBC collection, EBC volume increases linearly with the time of collection. Approximately 1–2 mL of EBC may be collected during the period of about 10 minutes (from 40 to 300 mL/min, average ~100 mL/min) (17).
Dilution of EBC
EBC contains droplet with large amounts of water vapour and traces of non-volatile solutes. Dilution of EBC is much greater than dilution of RTLF (25), since RTLF is diluting during condensation (Figure 2B). Although some methods have been developed to estimate the degree of EBC dilution, i.e. determination of urea, total cation concentration and of conductivity of lyophilized EBC (26), the exact degree of EBC dilution is still unknown. According to Effros, non-volatile constituents are diluted about 1:12,000 (26)
Contamination of EBC
Contamination of EBC occurs due to possible saliva and oropharingeal content leaking into the sample. Prevention of salivary contamination may be achieved by the use of EBC collectors with a mouthpiece and a two-way non-rebreathing valve. As saliva contains many specific biomarkers (proteins, lipids, carbohydrates), appropriate measures to prevent its leaking into EBC must be applied (collector selection, periodic swallowing of saliva during collection) (27). Determination of amylase catalytic concentration in EBC is used to determine the extent of salivary contamination and for rejection of unsuitable specimens of EBC. Bacterial flora from pharingo-oral tract may increase nitrite concentration in EBC (28). Bacterial contamination should be prevented by mouthwash with chlorhexadine before EBC collection.
Recently, fractionation of exhaled breath was used to reduce the contribution of upper airways (influence of environmental factors on upper airways) and to increase the contribution of bronchial and alveolar tracts on the EBC content, e.g. malondialdehyde (29).
Sample handling - pre-analytical procedures
Gas standardization of EBC
The value of pH is not stable due to the effect of the atmosphere and exhaled CO2. On gas standardization, i.e. deareation CO2 is removed from the sample, thus reducing the effect of CO2 on pH measurement (30). Gas standardization may be performed in more ways, e.g. by bubbling argon, partial CO2 pressure of 5.33 kPa and helium through the EBC samples. pH can be determined in EBC before (31) or after gas standardization (32). On each inspiration at rest, 10% of air in the lungs is exchanged by atmosphere air (33), where pO2 = 21 kPa and pCO2= 0. The content of CO2 and O2 is different in lungs and in atmosphere. pO2 in the inspired air is about 20 kPa and in the alveoles about 14.7 kPa, whereas pCO2 in the alveoles is about 5.3 kPa. In the exhaled air, pO2 is about 15.9 kPa and pCO2 is about 4.2 kPa (33). During the procedure of gas standardization of EBC, pH rises to a point at which stable reading is possible. In healthy individuals, pH of gas standardized EBC ranges between 7.4 and 8.8 (34), and in asthmatics pH ranges between 7.58 and 7.75 before therapy and between 7.67 and 7.91 after the therapy with salbutamol, respectively (35).
Extraction methods for some analytes from EBC
For chromatographic methods of some analytes in EBC (e.g. leukotrienes), their extraction/concentration is required. These procedures increase analytical sensitivity in the determination of analytes in EBC. These methods include immunoaffinity extraction, IAE (36), solid phase extraction, SPE (37), solvent extraction, SE (38) and lyophylization (39). IAE include separation of compounds with specific antibodies. By the SPE dissolved or suspended compounds are separated from other compounds in the liquid mixture based to their chemical and physical properties. SE is used to separate components according to their relative solubility in water and organic solvent. These extraction methods are performed for sample purification prior to chromatography analysis. Consequently, they reduce interferents from the EBC matrix that can influence the result. Lyophilization, also known as freeze drying process of dehydratation, may be used for concentrating non-volatile or semi-volatile compounds dissolved in EBC (39). In that way, EBC may be stored without refrigeration for long period (40). In addition, process of EBC lyophilisation removes volatile solutes, e.g. NH3 (41), which may influence pH or conductivity of EBC. An EBC sample lyophilized and then resuspended gives a 20-fold concentration of biomarkers (42). Lyophilisation may improve reproducibility in determination of some biomarkers (17).
EBC storage
Although EBC can be analyzed immediately after collection, samples are usually stored at low temperature until analysis (17), since some EBC constituents (H2O2, pH) are instable at room temperature. EBC can be stored at different freezing temperatures, i.e. from -20 oC (34) to -80 oC (17). However, freeze-thaw cycles should be avoided (2,3).
Analytical phase
Analytical methods
Apart from preanalytical issues of EBC, its analysis is very important methodological issue, because conventional assays must be adjusted to very low concentrations of biomarkers in EBC. The problem is more conspicuous in immunoassays, especially in commercial immunoassay kits, that neither are not validated for such low concentrations nor for the matrix of EBC sample (27). So, these conventional methods are affected by poor sensitivity and specificity.
Usually, methods for the analysis of EBC samples were traditional analytical methods for the analysis of other biological samples, and these methods are scheduled for the single-analyte determination, e.g. pH-metry, biosensors (43), spectrophotometry (44,45), spectrofluorimetry (46), enzymatic assay (47), immunoassays (ELISA, immunoluminometry, radioimmunoassay – RIA, immunosensors) (2). Immunoassays need to be validated with reference analytical methods such as mass spectrometry or high performance liquid chromatography (HPLC), that enable a precise determination of the concentrations of the different markers in EBC (4) (Table 1).
Recently, multi-analyte methods were introduced for the qualitative and quantitative analysis of EBC. These methods include two-dimensional protein gel electrophoresis (2-D PGE) (4) and micro-analysis of protein spots by chromatography. Chromatography methods are gas chromatography (GC), high performance liquid chromatography (HPLC), mass spectrometry (MS), liquid chromatography – mass spectrometry (LC-MS), gas chromatography – mass spectrometry (GC-MS) (48), high performance liquid chromatography – tandem mass spectrometry (HPLC-MS) (49), multi-parameter chemical sensors, i.e. chromatography differential mobility spectrometry (GC/DMS) (50), electrospray ionization differential mobility spectrometry (ESI/DMS) (51). All these multi-analyte methods make it possible to recognize parlar components in nanomolar concentrations. However, contaminations (even in nanograms) may cause different results.
Unfortunately, despite numerous investigations, analysis of EBC has limitations regarding optimization and validation of quantitative analytical procedures. Therefore, it is not possible to make a comparison of results from different laboratories (46). Determination of nitric oxide (NO) metabolites is based on spectrophotometric methods (2). H2O2 is measured spectrophotometrically, spectrofluorimetrically (52) or by the use of an automated amperometric biosensor (53). Eicosanoids in EBC are measured by different techniques, including immunoassays, chromatography and mass spectrometry; the latter method is considered as reference method (2). So, isoprostanes may be determined by using GC-MS; prostanoids by RP-HPLC (reversed phase-high performance liquid chromatography) and GC-M. For determination of leukotrienes HPLC or LC-MS may be recommended. LC-MS is considered as the ‘gold standard’ method for simultaneous determination of different isoprostanes (39). In addition, LC-MS is an ideal for the simultaneous determination of various purines (54). Methods for the determination of proteins in EBC depend on the concentration of the particular protein in EBC. Hence, total proteins may be measured spectrophotometrically (19), cytokines by using enzyme immunoassay 2-D PGE (4) and cytokeratins and other proteins present in nanomolar concentration by the use of HPLC-MS (55). Although pH-value was measured with different pH-electrodes or biosensors, Ross-type pH electrodes should be used for the determination of pH in EBC (45), since the EBC matrix has low ionic strength.
Recently, so called “omic science”, especially metabolomics and metabonomics, were introduced in the analysis of EBC. Metabolomics enables to look at genotype-phenotype relationships and genotype-envirotype relationship. Metabonomics may identify and quantify the metabolic fingerprint of EBC in different clinical sets of data. The methods that are used in metabolomic/metabonomic analysis are MS (detection in femtomoles), nuclear magnetic resonance (NMR) based spectroscopy (detection in micromoles) (56).
Method optimization/validation
Optimization and validation of conventional methods applied for EBC analysis are problematic, especially for the very low concentrations of biomarkers, since the coefficient of variations (CV) is much higher due to nonlinearity of the assay in that low range (57). All results in the low range are based on interpolation, and the determination of the lower limit of detection (LOD) and lower limit of quantification (LOQ) are disputable. In addition, validation of analytical methods for EBC analysis depends on the standardization of preanalytical phase. Analysis of EBC by using conventional analytical methods is more useful for qualitative than for quantitative analysis of EBC (46). Immunoassays are sensitive, but lack in specificity. Conventional techniques must be compared with reference methods, i.e. chromatographic techniques and mass spectrometry, methods that have satisfactory precision, accuracy, LODs, LOQs (58).
Limit of detection and quantification for analytes in EBC
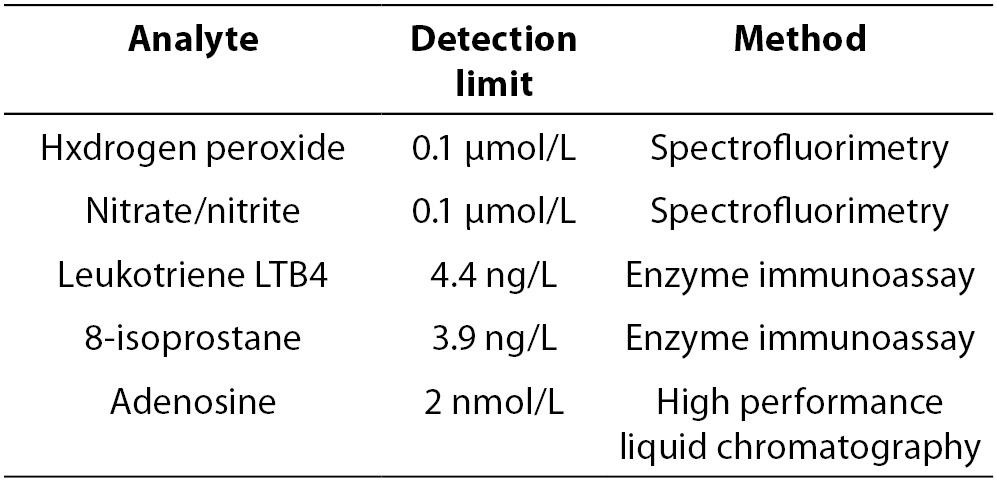
LOD defines the lowest quantity of a substance that can be distinguished from the blank value (zero, absence of particular substance) in the linear range of the assay, where the value of CV is known. LOQ presents the lowest quantity of the substance that can be measured. It is not easy to define the LODs and LOQs of EBC constituents, since native EBC sample is extremely diluted solution and most biomarkers are present at very low concentrations. Therefore, LODs and LOQs of EBC constituents depend on the pre-treatment procedures of EBC (preconcentration, i.e. lyophilisation). EBC preconcentration is necessary to improve LODs and LOQs values. In addition, LOD of particular component depends on the method used to determine its value (Table 2). In general, spectrophotometric methods and immunoassays have higher detection limits (detection at micromolar levels) than the chromatographic methods (detection at nanomolar and picomolar levels) (Table 2) (3,55).
Table 2. Detection limits for some analytes in exhaled breath condensate (19).
Data handling
Normalization factor for analytes in EBC
To account for the fluctuation of constituents in extremely diluted biological samples, laboratory medicine specialists try to normalize (i.e. adjust) the level of particular analyte using the marker with stable concentration. A normalization factor is defined as the ratio between two analytes in the same body fluid. Some authors (26) have tried to develop normalization factor, e.g. concentration of urea, cation concentration and osmolality and conductivity after lyophilisation. As the normalization factor must have the same physical and chemical characteristics (solubility, volatility) as the measured constituent, a universal normalization factor is not yet defined (59,60). Urea might be the normalization factor to control intra-individual variability of different analytes in EBC samples (59,60).
Post-analytical phase
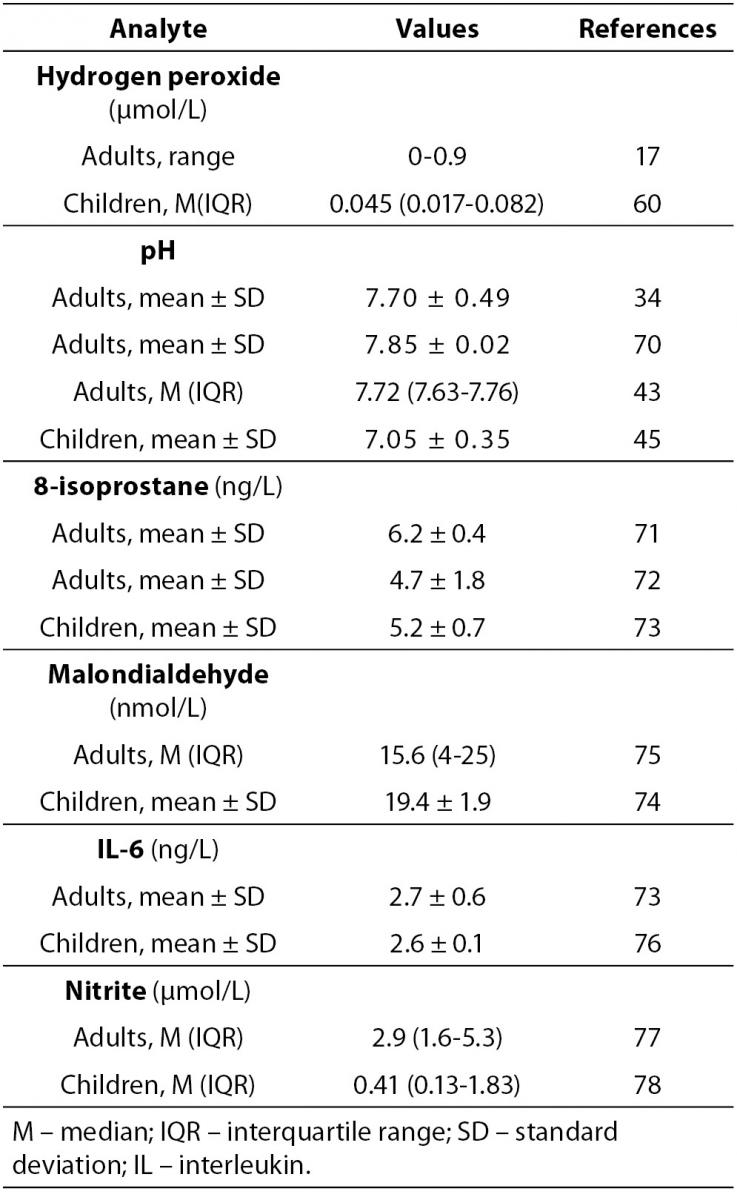
EBC is a matrix that contains large number of mediators that can be used as biomarkers of respiratory diseases. Biochemical biomarkers in EBC are indicators of health condition or indicators of a pathogenic biological process in respiratory system, particularly in lower respiratory tract. The interpretation of the values of the particular biomarker or biomarkers pattern in EBC samples is the final goal of EBC analysis. Laboratory medicine specialists and clinicians have to co-operate in this field of science. Values of EBC constituents have been extensively investigated in healthy subjects as well as in patients with respiratory diseases, since these diseases are associated with alterations of the RTLF composition (59). The majority of investigations refer to H2O2 (2,3,17,59), pH (2,3,19,30,61), nitric oxide (NO) (2,3,19) and its metabolites (2,3,62,63), purines (3,64,65), various eicosanoides (prostanoides, leukotrienes) (2,3,66) and proteins (interleukins, cytokines, cytokeratins) (42,67), electrolytes (68), urates (69). Special issue is referred on mebolomics, i.e. the analysis of multiparametric metabolic data (56). For some biomarkers, values are determined for healthy subjects, and some of them are shown in Table 3 (H2O2 (17,60), pH (34,43,46,70), 8-isoprostane (71-73) malondialdehyde (74, 75), IL-6 (76), nitrite (77, 78).
Table 3. Concentration of biomarkers in exhaled breath condensate in healthy adults and children.
Reference values for key biomarkers in EBC
Determination of reference values of particular analytes in EBC and selection of different disease-related biochemical biomarkers are integral part of post-analytical phase of the EBC analysis (Table 1, Table 3). However, reference values for EBC constituents are not established, since some analytical problems are not solved. Concentrations of some biomarkers in EBC obtained in healthy subjects, adults and children, are presented in Table 3. These values derived from the literature data in investigations of biomarkers in patients with respiratory diseases, where healthy subjects had participated as control groups. Future studies are needed to establish the reference values of EBC biomarkers (79).
Clinical use and interpretation of biomarkers in respiratory diseases
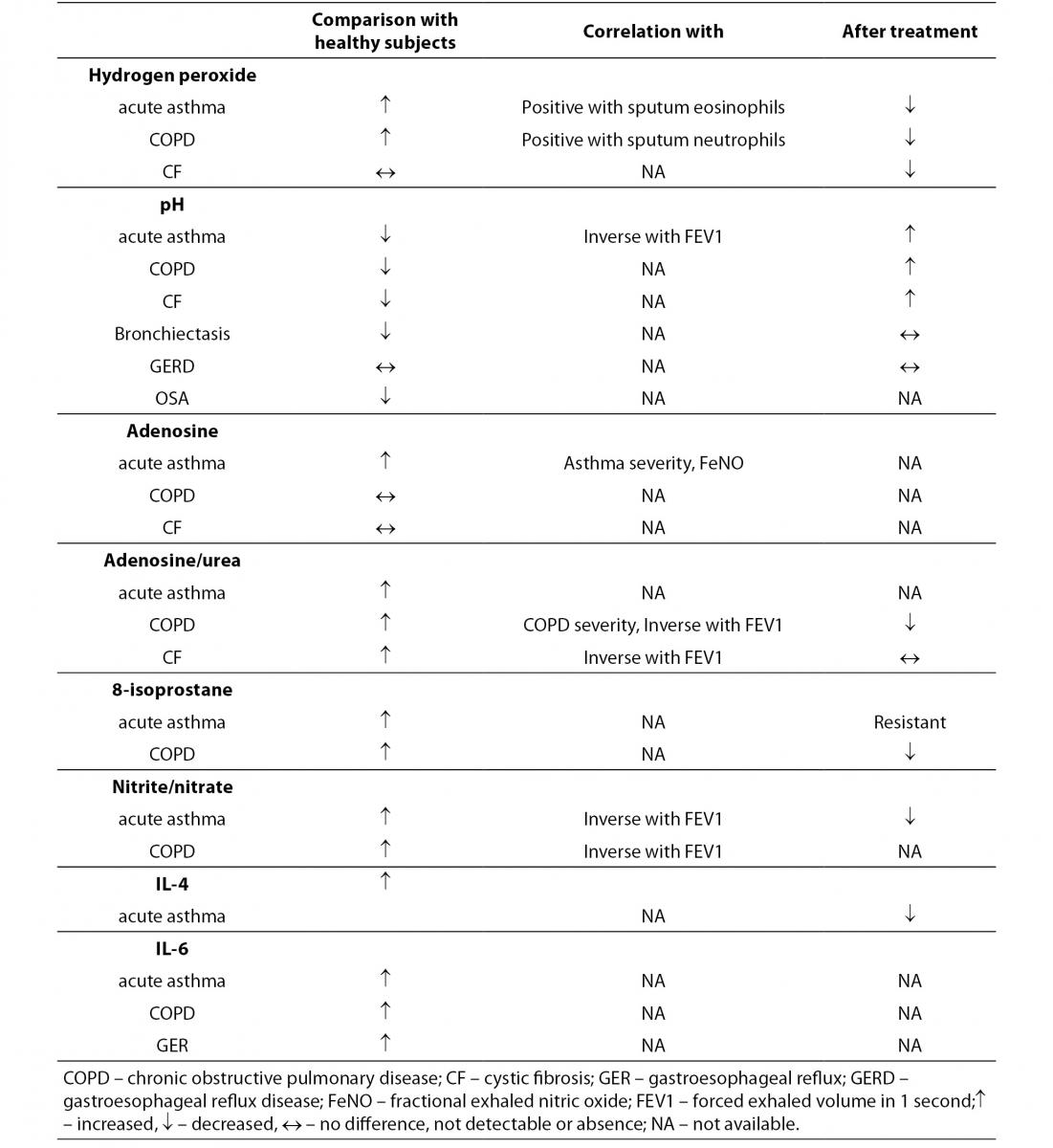
A number of investigations study the changes of biomarkers in different pulmonary diseases (Table 1, Table 4). The list of states and diseases for which EBC analysis is used include asthma (2,3,17,44,69,73, 74,77,89,90,95), COPD (2,3,42,47,54,71-73,77,83,86, 96), gastroesophageal reflux disease (GERD) (2,3,45), obstructive sleep apnea (OSA) (80,94), pulmonary fibrosis (81), pneumonia (4,17), cystic fibrosis (7,17,76,92), adult respiratory distress syndrome (ARDS) (3,17,93), non-respiratory diseases (82,84) (Table 1).
Table 4. Biomarkers in EBC in relationship with clinical studies (adapted according to reference 4).
Increased values of H2O2 in EBC were found in healthy smokers (17), in patients with asthma (19,60), COPD (17,93), cystic fibrosis (7) bronchiectasis (17), ARDS (17), as well as in non-respiratory disease, e.g. systemic sclerosis (84). In addition, day to day intra-subject CV of 43% in healthy subjects has been reported (85).
Significant decrease in EBC pH has been reported in patients with acute exacerbation of asthma (17) and in COPD (86). The term ‘acid stress’ describes a shift in pH toward the more acid values (87). In asthmatics, pH returned back higher values after successful anti-inflammatory treatment with steroids (17,60). Furthermore, pH levels were related to eosinophilic (in asthmatics) or neutrophilic (in patients with COPD) inflammation of the airways (19).
Concentrations of NO and NO-related compounds were found to be significantly higher in asthma (63). In patients with COPD, literature data of nitrite/nitrate levels are not consistent. Some studies have indicated increased levels (88), and some studies reported unchanged levels of nitrite/nitrate in comparison with healthy controls (62). Children with CF are reported to have decreased levels of NO and increased levels of nitrite in EBC (63). It seems that NO production in these patients is trapped by mucus in the airways. Therefore, the determination of nitrite might be a better biomarker of NO production in patients with CF.
Leukotrienes, Cys-LTs and LTB4, were reported to be increased in asthmatic patients as compared with healthy subjects (89). Prostaglandine PGE2 levels were found to be increased in EBC in patients with COPD (83). In these patients, LTB4 increased in the stable state. During exacerbation, the concentration of LTB4 was markedly higher than in the stable phase of disease, and after antibiotic treatment LTB4 levels were decreased (71). In patients with CF concentration of LTB4 were increased (76).
8-isoprostane concentration was found to be elevated in asthma (90), COPD (83), interstitial lung disease (91), CF (92), ARDS (93), OSA (94). In patients with acute exacerbation of asthma or COPD, 8-isoprostane concentrations were found to be decreased after anti-inflammatory medications (17).
Concentrations of interleukins are changed in some pulmonary diseases in comparison with healthy subjects, e.g. IL-4 levels were increased, and interferon-γ levels were decreased in ascthmatic subjects (95). Furthermore, medication with steroids resulted with decrease of IL-4 and increase of interferon-γ concentration. Concentrations of IL-6 were increased in patients with OSA (94), COPD (96), CF (76), lung cancer patients (97), and in cigarette smokers (98) (Table 4).
Outlook
We must be aware that EBC analysis is still used as a research tool only. Limitations referred to pre-analytical, analytical and post-analytical phases of EBC analysis are numerous. The major limitation is enormous dilution of the RTLF, and consequently, low concentrations of EBC constituents, with detection levels close to the limits of analytical methods. Furthermore, methods for single-analyte determinations lack in sensitivity. Today, the interpretation of the results obtained is somewhat doubtful since reference values are not established. Therefore, research and standardization of all the phases of the analytical process should be continued to allow appropriate interpretation of the results obtained. The application of multi-analyte EBC analysis in the field of metabonomics, proteomics, and genomics (99,100) has not been fully explored. Metabolomic/metabonomic based analyses of EBC have shown the possibility to discriminate between healthy and asthmatic subject, and furthermore to characterize asthma sub-phenotypes. Some efforts are made to investigate DNA as a biomarker for lung diseases (2). However, the results are dissonant, since some authors have detected DNA, and some authors failed to detect DNA in EBC. Our attempt to detect DNA in EBC was unsuccessful, too.
Hopefully, when all pre-analytical, analytical and post-analytical requirements are met, EBC biomarkers can be selected, and EBC analysis can be used in clinical practice, in the diagnosis and, even in the longitudinal follow-up of patients (monitorig of therapy effects), resulting in better outcome of disease.
Acknowledgement
The results presented were obtained in the scope of a scientific project 277-2770966-0965, entitled Exhaled Breath Condensate as a Source of Lung Disease Biomarkers in Children, carried out with support from the Ministry of Science, Education and Sports of the Republic of Croatia.