Effect of duration of diabetes mellitus type 1 in children and adolescents on urinary N-acetyl-β-D-glucosaminidase excretion
Jasminka Matica
[*]
[1]
Višnja Rački-Čargonja
[1]
Štefica Dvornik
[1]
Introduction
Renal damage is serious complication in diabetes mellitus. If diabetic nephropathy is diagnosed by microalbuminuria, proteinuria or decreased creatinine clearance, little can be done to prevent the progressive course of renal failure (1). There is a need for earlier indicators of renal structural and functional changes which may be prevented by strict glycemic control.
A number of studies have shown that early tubular alteration due to peritubular microangiopathy may precede glomerular cha nges and play a role in the initiation of diabetic nephropathy (2). Increased urinary excretion of enzymes that originate from proximal renal tubules were found in patients with diabetes mellitus type 1 as result of tubular injury: N-acetyl-b-D-glucosaminidase (NAG), alanine-aminopeptidase, alkaline phosphatase, urinary dipeptidyl aminopeptidase IV (3). Activity of these tubular markers is increased in urine before the appearance of microalbuminuria (4).
NAG (EC 3.2.1.30) is a high molecular-weight lysosomal enzyme. It is widely distributed in many tissues and body fluids. Urinary NAG is of renal origin (5) as it cannot pass through glomerular membrane due to its high molecular weight (130 000 to 140 000 D). It is released from cells as a consequence of physiological exocytosis (6) or breakdown of cells (7). NAG is distributed along the whole nephron but mainly in renal proximal tubulesand it is considered to be a non-invasive early marker of renal tubular damage. Urinary NAG activities are elevated in pyelonephritis (8), reflux nephropathy (9), hypertension (5)and rheumatoid arthritis, exposure to nephrotoxic drugs, environmental pollutants like mercury (10), cadmium (11) and in workers occupationally exposed to trichloroethylene (12). It can be a useful parameter in patients with tubulo-interstitial nephropathies to distinguish acute from chronic disorders. In acute damage of the tubule system, NAG activity of more than 20 U/L (15 U/g creatinine) can be measured (13).
Urinary NAG is an early predictor of development of diabetic nephropathy, it increases before the appearance of microalbuminuria (14) in diabetes mellitus type 1.
Schultz et al. (15) have shown that raised activity of urine NAG in subjects under sixteen years of age are related to duration of diabetes type 1 and concentration of glycated hemoglobin. Mocan et al (16) examined 54 adults with diabetes mellitus type 2 and observed that NAG activity begins to rise in the third year of diabetes, reaches a plateau between 3–10 years, and rapidly increases after the 10th year.
Kavukçu (17) showed that urinary NAG activity is related to glucose level in blood and Kordonouri et al. (18) found that patients with diabetes type I onset in childhood showed significantly higher activities of NAG compared to those with diabetes onset after the age of sixteen years, and concluded that more frequent impairment of tubular function was observed in young patients with diabetes onset in childhood maybe due to non-optimal glycemic control in this population.
The aim of this study was to examine whether there are differences between urinary NAG activity between children and adolescents with diabetes mellitus type 1 and healthy subjects. Beside that, we want to examine whether urinary NAG activity change in relation to duration of the disease.
Materials and methods
Subjects
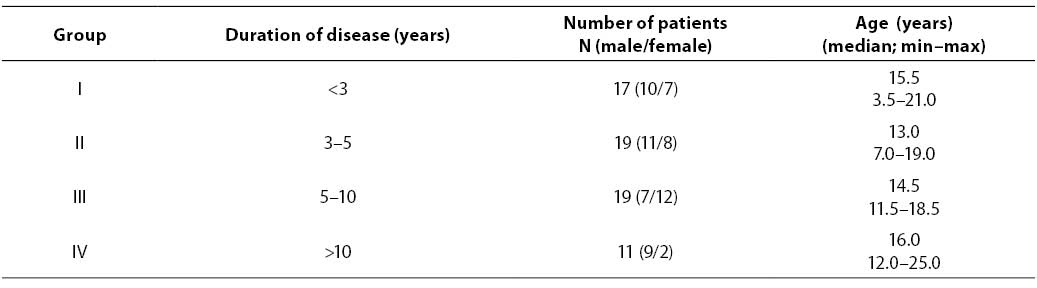
The study included 66 children and adolescents with diabetes mellitus type 1 monitored during three years in Kantrida Children’s Hospital, Rijeka. There were 37 males and 29 females; median age: 14.5 (3.5–25). In the control group, there were 68 individuals (29 males and 39 females) without renal disease and diabetes; median age 11.0 (0.5–27). The diabetic patients were classified in four groups according to the duration of diabetes. (Table 1).
Table 1. Characteristics of groups of diabetic patients
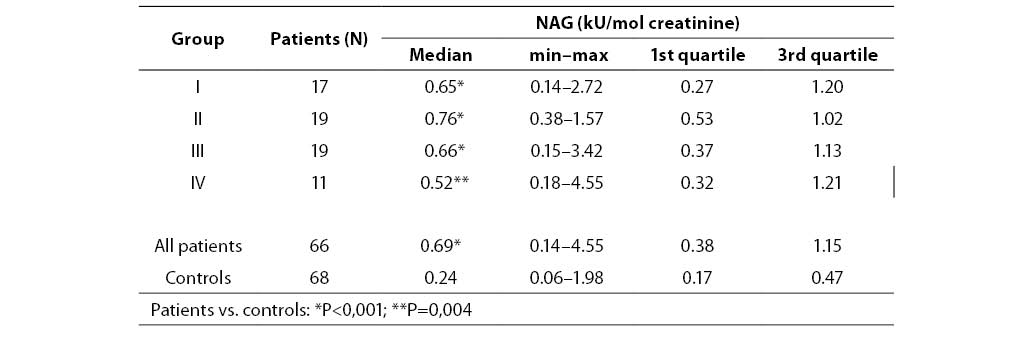
Table 2. Urinary NAG activities in diabetic patients and controls
Samples
Random urine samples were collected from each subject without additives, centrifuged 10 min at 800 g and supernatants were kept at –20 oC until NAG activity analysis which was performed once a week. NAG activity is stable over 7 days at 4 oC and –20oC (19).
Fresh urine samples were used for determination of urinary creatinine concentration and albumin.
Whole blood was collected with EDTA as anticoagulant for determination of HbA1c.
Methods
Urinary NAG activity was determined spectrophotometrically by commercial test kit (Boehringer Mannheim, Germany) with 3-cresolsulfonphthaleinyl-N-acetyl-b-D-glucosaminide as substrate (20). Urinary creatinine concentration was measured using modified Jaffe reaction (21) by home made reagent. In order to minimize the effect of urine flow rate on urinary enzyme activity of NAG, results are expressed as the ratio of enzyme activity to urine creatinine concentration (kU/mol creatinine).
Glycated hemoglobin concentration was determined by ion exchange chromatography (Bio Systems, Spain).
Urinary albumin concentration was determined by radial immunodiffusion (Behring, Germany).
Statistical analysis
The Kolmogorov-Smirnov test was applied to test for a normal distribution Comparison of urinary NAG activity between entire diabetic group and controls was made by unpaired Mann-Whitney test. Comparison between four diabetic groups and control group was made by Kruskal-Wallis test (P<0.05) and post hoc by unpaired Mann-Whitney test (P<0.005).
Spearman’s correlation coefficient between urinary NAG activity in diabetic patients and duration of disease was calculated.
Comparison between four diabetic groups for urinary albumin concentration and glycated hemoglobin concentration was made by Kruskal-Wallis test (P<0.05).
Results and discussion
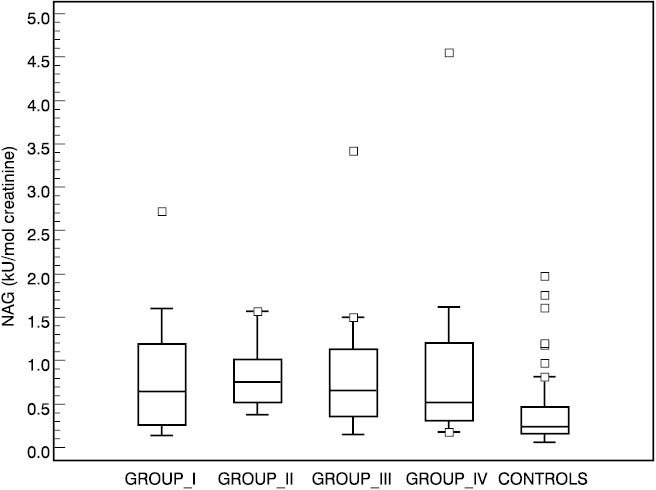
Urinary NAG activities in four groups of diabetic patients and in the control group are shown in Table 2. Significantly higher values were found in all four diabetic groups compared to the control group (group I P=0.001, groups II and III P<0.001 and group IV P=0.004 compared to controls). The lowest median for NAG was in group IV (0.52, 0.18–4.55 kU/mol creatinine) which comprised patients with the longest duration of diabetes mellitus, and the highest median for NAG was established in group II (0.76, range 0.38–1.57 kU/mol creatinine). There was no significant difference in NAG excretion among any diabetic groups. Box-Whisker plot presents median, quartiles, minimum, maximum and outliers of each group. Distribution of urinary NAG activities in four subgroups and controls is presented in Figure 1.
Figure 1. Box-Whisker plots of urinary NAG activities in controls and patient subgroups.
Urinary NAG activity of all four groups was significantly higher compared to controls regardless of the duration of diabetes. On the other hand, there was no correlation between the four diabetic groups and disease duration (r= –0.017 P=0.892).
Hsiao et al. (22) examined thirty-one children with diabetes mellitus type 1 and found significantly higher urinary NAG activity in four diabetic groups (the groups were divided in the same manner as ours, according to duration of diabetes) compared to the control group, but there was no statistically significant difference among any two of these four groups. Lorini et al. (23) and Mungan et al. (24) also did not find correlation between urinary NAG activity and duration of diabetes type 1 in young diabetic patients.
Urinary NAG is related to glucose concentration in blood (17)and may reflect metabolic control for patients with diabetes mellitus, activity of the disease and the severity of damage.
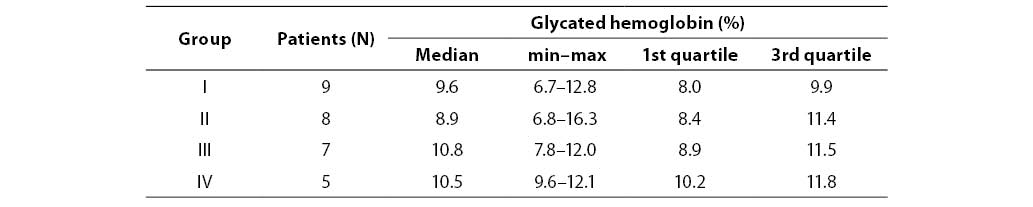
Our patients from all four diabetic groups had similar concentrations of glycated hemoglobin, which indicated that they had similar levels of glycemia. There was no significant difference between any two of the four diabetic groups in glycated hemoglobin (P=0.574) as presented in Table 3. Significantly higher activities of urinary NAG in children and adolescents with diabetes type 1 compared to controls indicate that these diabetic patients had tubular damage which was possibly a consequence of poor glycemic control (the median of glycated hemoglobin levels was 8.9% or higher in all four groups).
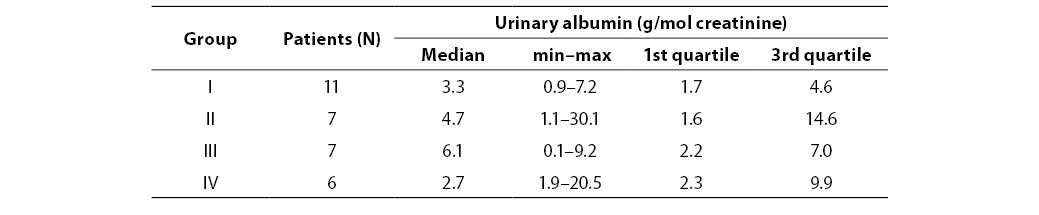
Table 3. Urinary albumin concentration in patients
There was no statistically significante difference in urinary albumin concentration between diabetic groups. Table 4 presents urinary albumin values in four groups of diabetic patients.
Table 4. Glycated hemoglobin concentration in patient subgroups
It is possible that there was no difference between groups in urinary NAG excretion because of their similar level of glycemia and, as a consequence, similar degree of renal damage.
There are many factors that affected urinary NAG excretion so it is impossible to make definitive conclusions about reason consequence relation between increased NAG activity and impairment of tubular function in diabetes mellitus. Further studies on larger study groups are needed to provide those answers.
Acknowledgments
We thank Katarina Cvijović, PhD, who provided us with the data about diabetic patients and Lidija Bilić-Zulle, MSc, who gave us useful advice for statistical analysis.
References
1. Turecky L, Uhlikova E. Diagnostic significance of urinary enzymes in nephrology. Bratisl Lek Listy 2003;104:27-31.
2. Nuyts GD, Yaquoob M, Nouwen EJ, et al. Human urinary intestinal alkaline phosphatase as an indicator of S3-segment-specific alterations in incipient diabetic nephropathy. Nephrol Dial Transplant 1994;9:377-81.
3. Ishii N, Ogawa Z, Itoh H, Ikenaga H, Saruta T. Diagnostic significance of urinary enzymes for diabetes mellitus and hypertension. Enzyme Protein 1994-95;48:174-82.
4. Hong CY, Chia KS. Markers of diabetic nephropathy. J Diabet Compl 1988;12:43-60.
5. Hashimoto R, Adachi H, Nishida H, Tsuruta M, Nomura G. Serum N-acetyl-b-D-glucosaminidase activity in predicting the development of hypertension. Hypertension 1995;35:1311-4.
6. Price RG. Measurement of N-acetyl-b-glucosaminidase and its isoenzymes in urine methods and clinical applications. Eur J Clin Chem Clin Biochem 1992;30:693-705.
7. Welman E, Selwyn AP, Peters TJ, Colbeck JF, Fox KM. Plasma lysosomal enzyme activity in acute myocardial infarction. Cardiovasc Res 1978;12:99-105.
8. Belli A, Scalercio F, Martino F, et al. Evaluation of N-acetyl-beta glucosaminidase in upper and lower urinary tract infections in childhood. Clinical study of 168 children. Minerva Pediatr 1996;48:503-7.
9. Konda R, Sakai K, Ota S, Takeda A, Orikasa S. Followup study of renal function in children with reflux nephropathy after resolution of vesicoureteral reflux. J Urol 1997;157:975-9.
10. Cárdenas A Roels H, Bernard AM, et al. Markers of early renal changes induced by industrial pollutants. I Application to workers exposed to mercury vapour. Br J Ind Med 1993;50:17-27.
11. Roels H, Bernard AM, Cárdenas A, et al. Markers of early renal changes induced by industrial pollutants. III Application to workers exposed to cadmium. Br J Ind Med 1993;50:37-48.
12. Green T, Dow J, Ong CN, et al. Biological monitoring of kidney function among workers occupationally exposed to trichloroethylene. Occup Environ Med 2004;61:312-7.
13. Ivandić M, Hofmann W, Guder WG. Development and evaluation of urine protein expert system. Clin Chem 1996;42:1214-22.
14. Jones AP; Lock S; Griffiths KD. Urinary N-acetyl-beta-glucosaminidase activity in type I diabetes mellitus. Ann Clin Biochem 1995;32:58-62.
15. Schultz CJ, Dalton RN, Neil HAW, Konopelska-Bahu T, Dunger DB. Markers of renal tubular disfuntion measured annually do not predict risk of microalbuminuria in the first few years after diagnosis of Type I diabetes. Diabetologia 2001;44:224-9.
16. Mocan Z; Erem C; Yildirim M; Telatar M; Deger O. Urinary beta 2-microglobulin levels and urinary N-acetyl-beta-D-glucosaminidase enzyme activities in early diagnosis of non-insulin-dependent diabetes mellitus nephropathy. Diabetes Res 1994;26:101-7.
17. Kavukçu S, Soylu A, Türkmen M. The clinical value of urinary N-acetyl-b-D-glucosaminidase levels in childhood age group. Acta Med Okayama 2002;56:7-11.
18. Kordonouri O, Kahl A, Jorres A, et al. The prevalence of incipient tubular dysfunction, but not of glomerular dysfunction, is increased in patients with diabetes onset in childhood. J Diabet Compl 1999;13:320-4.
19. Hofmann W, Guder G. A diagnostic programme for the quantitative analysis of proteinuria. J Clin Chem Clin Biochem 1989;27:589-600.
20. Noto A, Yasunao O, Mori S, et al. Simple, rapid spectrophotometry of urinary N-acetyl-b-D-glucosaminidase, with use of a new chromogenic substrate. Clin Chem 1983;29:1713-6.
21. Cook JGH. Creatinine assay in the presence of protein. Clin Chim Acta 1971;32:485-6.
22. Hsiao PH, Tsai WS, Tsai WY, et al. Urinary N-acetyl-b-D-glucosaminidase activity in children with insulin-dependent diabetes mellitus. Am J Nephrol 1996;16:300-303.
23. Lorini R, Scaramuzza A, Cortona L, et al. Increased urinary N-acetyl-beta-glucosaminidase (NAG) excretion in young insulin-dependent diabetic patients. Diabetes Res Clin Pract 1995;29:99-105.
24. Mungan N, Yuksel B, Bakman M, Topaloglu AK, Ozer G. Urinary N-acetyl-beta-D-glucosaminidase activity in type I diabetes mellitus. Indian Pediatrics 2003;40:410-4.