Introduction
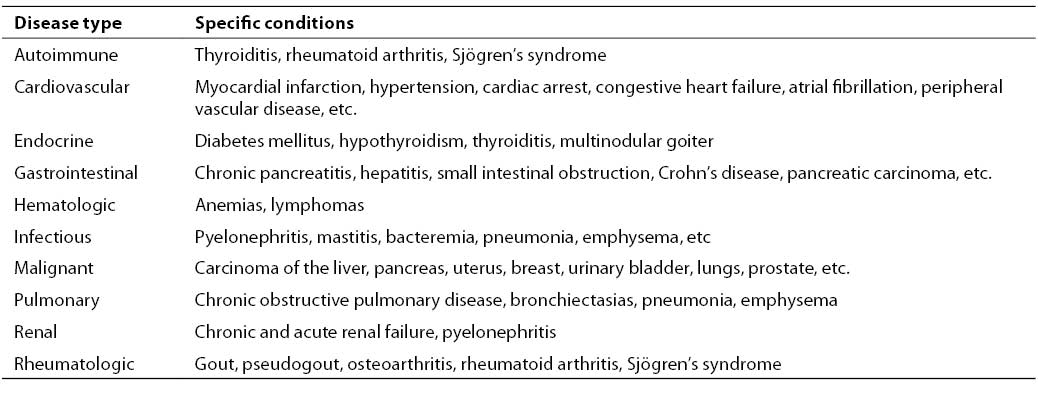
The presence of various macroenzymes in serum is one of the relatively rare causes of high enzyme activity. Numerous macroenzymes have been described in the literature (1-4), of which the occurrence of the macroenzyme amylase, first discovered in 1964 (5), is best characterized. Macroenzymes are normal serum enzymes (or isoenzymes), which form high molecular mass complexes by their polymerization, linking with other serum high molecular mass constituents, mostly immunoglobulins, or complex with cell membrane segments (1). Macroenzymes undergo a slower clearance rate due to their high molecular mass, thus accumulating in serum and enhancing the respective enzyme activity. These enzyme forms are as a rule detected in patients with continuously elevated serum enzyme activity that cannot be explained and is inconsistent with the general clinical picture. Therefore, macroenzymes may interfere with interpretation of the serum enzyme activity findings and thus lead to diagnostic and therapeutic errors. A failure to detect macroenzymes as the cause of unexplained elevation in the serum enzyme activity may result in the use of expensive, unnecessary and possibly invasive procedures in making an alternative diagnosis. For example, Weidner et al. (6) describe a 54-year-old female patient hospitalized on several occasions for isolated high activity of aspartate aminotransferase (AST). She had undergone repeat liver biopsies and consultations with neurologists, endocrinologists and gastroenterologists before the high enzyme activity was eventually ascribed to the presence of macroenzyme. The occurrence of the macroenzyme forms of some enzymes has been associated with certain autoimmune disorders such as rheumatoid arthritis, IgA immunodeficiency, systemic lupus erythematosus (SLE) and ankylosing spondylitis (7-9), with some malignant diseases such as carcinoma of the stomach, breast and prostate, as well as some accidental conditions (10,11). A survey of medical records between 1988 and 1990 at Mayo Clinic revealed the presence of various macroenzymes in 42 patients (4). The diagnoses recorded in patients with detected macroenzymes are listed in Table 1. It should be noted that patients with macro creatine kinase (macroCK) and macro lactate dehydrogenase (macroLD) were older than 60, whereas those with macroAST were younger.
Table 1. Diagnoses in 42 patients with presence of macroenzymes (4)
Generally, the presence of macroenzymes is considered to be suggested by (a) the absence of symptoms in association with high enzyme activity; (b) the presence of symptoms atypical for high enzyme activity; and (c) isolated, continuously elevated enzyme activity showing no variation with time. According to literature data, the length of the macroenzyme presence in serum may vary from days, months and years through permanent presence. Techniques such as electrophoresis, immunoinhibition, immunoprecipitation and chromatography are usually employed to detect and define these complexes. Quite frequently, a combination of techniques has to be used.
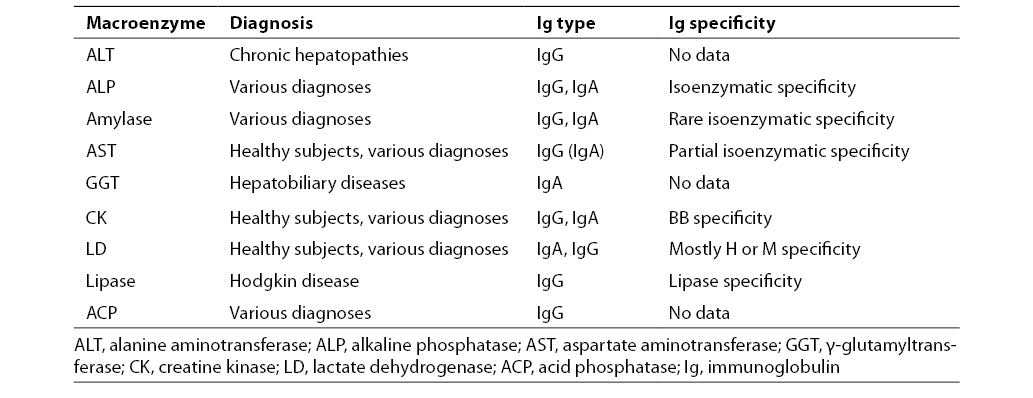
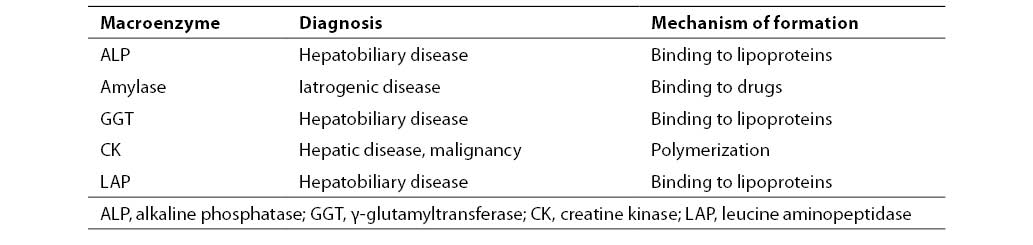
Macroenzymes are generally classified into two main groups: macroenzymes type 1 (Table 2) and macroenzymes type 2 (Table 3).
Table 2. Macroenzymes type 1 isolated in patient sera (12)
Table 3. Macroenzymes type 2 isolated in patient sera (12)
Macroenzymes type 1
This type includes the macroenzymes formed by serum enzyme (or more frequently particular isoenzyme) linking with specific immunoglobulins, mostly IgG and IgA class, and less frequently IgM class. The reason for the formation of the enzyme-immunoglobulin complexes remains unknown. Although some proteins undergo nonspecific linking to immunoglobulins, all relevant analytical procedures indicate the enzyme-immunoglobulin complex to have the characteristics of Ag-At complex (1,2). The 2:1, 1:1 or 1:2 immunoglobulin to enzyme (isoenzyme) ratios have mostly been described in these complexes, resulting in the respective macroenzyme molecular mass variation. The specific enzyme binding site is mostly on the Fab and F(ab1)2 fragment of the immunoglobulin molecule (13,14). The enzyme linking to the immunoglobulin Fab fragment stabilizes enzyme activity against high temperature, reduces the level of elimination, and influences kinetic parameters of the enzyme. In most cases, the enzyme linking to immunoglobulin has no or only a weak inhibitory effect on the particular enzyme activity (12). According to current concepts, the formation of complexes appears to be induced by structural immunoglobulin rather than enzyme molecule abnormalities (2).
Characteristics of some of this group macroenzymes are briefly presented below.
a) The alkaline phosphatase macroenzyme type 1 (macroALP) was first discovered in 1975 (15). Its prevalence has been estimated to 0.3%-0.4% of sera in which ALP isoenzymes are being determined. The bone or hepatic form of isoenzyme is mostly present in the complex, and is predominated with the IgG immunoglobulin class, usually l subtype, and less commonly k subtype. The macroenzyme molecular mass has been reported to range from 280 000 to 540 000, and is patient dependent. In patients with this type of macroALP, the activity of ALP usually shows a twofold increase, however, higher as well as normal activities have also been reported. The increased enzyme activity may persist for months or years. Like the majority of macroenzymes, the association of macroALP with particular diseases remains unclear, however, some studies suggest it to be more frequently associated with autoimmune disorders.
b) The amylase macroenzyme type 1 (macroamylase), a complex formed of one immunoglobulin molecule and one amylase molecule, shows a prevalence of 1.0% in the population with normal amylase activity and of 2.5% in patients with high amylase activity (16); however, a higher prevalence has also been reported (17). According to literature data, the immunoglobulin part of the complex includes IgAk, IgAl, IgGk or IgGl. The picture in patients with macroamylase present in serum is characterized by 1.5- to 8-fold serum enzyme activity and abnormally low amylase:creatinine clearance. Two cases of simultaneous occurrence of macroamylase and macrolipase in serum of patients with gluten enteropathy (17) and SLE (18) have been described.
c) The aspartate aminotransferase macroenzyme (macroAST) was first described in 1978 (19). Later on, this macroenzyme has been reported in some 20 patients with various diagnoses, most frequently acute and chronic hepatitis, malignant and autoimmune diseases, however, it was also found in healthy subjects (20,21). In most cases, the immunoglobulin component was IgG, less frequently IgA. The prevalence of macroAST in the general population is unknown. It is considered that the presence of macroAST should always be tested in case of asymptomatic elevation of AST activity (16).
d) The lactate dehydrogenase macroenzyme LD type 1 (macroLD) is more frequently present in some diseases such as neoplasms, liver disease and cardiovascular disease (22-24), however, it has also been reported in some burn patients (22) as well as in healthy subjects (25). In a study performed in a large number of patients, a 0.03% prevalence of macroLD and a threefold increase in LD activity on an average were found in subjects with macroLD detected in serum. The most common immunoglobulin components are IgG and IgA; a number of complexes with IgM, with IgG and IgM concurrence, and one case with IgG and IgA concurrence have also been described, with a varying prevalence of lambda and kappa light chains. The macroenzyme molecular mass mostly ranges between 420 000 and 490 000, however, complexes with Mr > 600 000 and < 1 000 000 have been reported. It has not yet been clarified whether LD linking to immunoglobulin corresponds to a specific Ag-At reaction, as there also are data on nonspecific interactions (26).
e) The isoenzyme CK-BB and IgG complex typically characterizes the creatine kinase type 1 macroenzyme (macroCK), although complexes formed of other isoenzymes and immunoglobulins may also occur (4). The reported prevalence of this macroCK type is 0.43%–1.2% (27). However, its prevalence depends on the method of determination, age, sex and disease characteristics. Total enzyme activity may rise 3- to 18-fold, or may be normal. The CK-2 to total CK activity ratio > 0.25 as a rule points to the presence of macroCK. On cellulose acetate electrophoresis, macroCK type 1 is localized in-between CK-MM and CK-MB (macroCK type 2 is localized more cathodically in comparison with CK-MM). The clinical relevance of macroCK type 1 has not yet been fully clarified, however, it has mostly been associated with autoimmune disorders. In their study, Galarraga et al. show, taking the example of macroCK, how the occurrence of macroenzymes may confound the interpretation of patient findings (28).
Macroenzymes type 2
In the group of macroenzymes type 2, most literature data refer to macroCK type 2, and to a lesser extent to ALP, g-glutamyltransferase (GGT), amylase, lipase, 5-nucleotidase and leucin aminopeptidase. This type is defined as a group of macroenzymes unbound to immunoglobulins. These are enzyme complexes formed by their polymerization (29) and linking to other serum components, e.g., hydroxyethyl starch (30), lipoproteins such as VLDL, LDL, lipoprotein-X (1), a2-macroglobulin (31), and cell membrane fragments, which is highly relevant in case of so-called membrane enzymes such as ALP, GGT, leucin aminopeptidase, LAP and 5-nucleotidase (3,32). The latter type of macroenzymes generally occurs in patients with hepatobiliary diseases, where bile acids play a major role in the formation of macroenzymes.
Besides macroCK type 2, which may lead to a false-positive diagnosis of myocardial infarction, this type of macroenzymes rarely causes difficulties in the interpretation of results in clinical enzymology (1,33). As these forms disappear from the circulation with improvement in the patient’s condition following appropriate therapy, indicating them to be of transient nature, they are believed to reflect the activity or dynamics of the disease. Therefore, this macroenzyme type may prove to be of clinical relevance for its potential role of a disease marker.
Hepatobiliary macroenzymes may be useful in differentiating extrahepatic from intrahepatic obstruction, for which there is no other satisfactory laboratory procedure available. As early as 1985, Wenham et al. found the complex GGT form activity to have a sensitivity of 88% and specificity of 96% in differentiating extrahepatic and intrahepatic obstruction (34). Furthermore, Turecky et al. believe that the assessment of GGT activity associated with VLDL+LDL lipoproteins may contribute considerably to the differentiation of chronic hepatopathies and malignant liver disease (35). A diagnostic sensitivity of 87% and specificity of 65% was demonstrated in differentiating chronic active hepatitis and liver cirrhosis.
MacroCK type 2
The prevalence of macroCK type 2 is reported to be 0.5%-3.7%. This macroenzyme type is considered to originate from mitochondria (oligomeric mitochondrial CK), and its occurrence in serum is generally associated with malignant lesions (in malignant disease, it shows a threefold incidence recorded for macroCK type 1) and liver disease (29,36). The more so, Wright and Liggett (27) recommend that noninvasive testing to rule out malignant disease, e.g., urinalysis, occult bleeding in stool, PSA, CA-125, CEA, mammography, radiological lung examination and pelvis ultrasonography be done in patients with elevated CK or CK-MB without evidence for cardiac or muscular disease. The occurrence of this macroenzyme has also been associated with a higher mortality rate (29,36). When present in children’s sera, macroCK type 2 is a marker of heart disease. One of the postulated mechanisms of macroCK type 2 association with malignant disease is direct enzyme release from malignant or necrotic cells (29). The occurrence of the macroenzyme need not always be associated with the increase in total CK activity, however, macroCK may interfere with some immunoinhibition procedures of CK-MB determination (2). Yet, it should be noted that macroenzymes CK should be considered in patients with CK-MB concentrations exceeding 50% of total enzyme activity, because values greater than 30% are rarely found even in patients with myocardial infarction.
The exact detection of macroenzyme CK requires procedures that enable determination of their molecular mass (chromatography, gradient gel electrophoresis). A number of screening tests for macroCK have been described (37).
Conclusion
Accordingly, awareness of the presence of macroenzymes and procedures for their determination should be an integral part of diagnostic work-up, in order to obviate the use of unnecessary expensive and invasive diagnostic procedures. The patient’s physician should be informed on the presence of a macroenzyme in the patient’s serum. These data should be entered in the patient’s medical records and history form. On the other hand, the patient should be properly reassured that the occurrence of this enzyme form requires no specific therapeutic intervention.
It should be noted that data on the possible diagnostic significance of the macroenzyme occurrence remain scarce, vague and questionable. This fact should not be neglected, but the information on the reasons for the presence of macroenzymes and on the mechanisms of their formation should be continuously collected and reconsidered. Indeed, future research into the formation and detection of macroenzymes may hopefully result in defining some new diagnostic markers.
References
1. Remaley AT, Wilding P. Macroenzymes: biochemical characterisation, clinical significance, and laboratory detection. Clin Chem 1989;35(12):2261-70.
2. Sturk A, Sanders GTB. Macroenzymes: prevalence, composition, detection and clinical relevance. J Clin Chem Clin Biochem 1990;28:65-81.
3. Turecky L. Macroenzymes and their clinical significance. Bratisl Lek Listy 2004;105(7-8):260-3.
4. Galasso PJ, Litin SC, O’Brien JF. The macroenzymes: a clinical review. Mayo Clin Proc 1993;68:349-54.
5. Wilding P, Cooke WT, Nicholson GI. Globulin-bound amylase: a cause of persistently elevated levels in serum. Ann Intern Med 1964;60:1053-9.
6. Eeidner N, Lott JA, Yale VD, Wahl RL, Little RA. Immunoglobulin-complexed aspartate aminotransferase. Clin Chem 1983;29:382-4.
7. Crofton PM, Kilpatrick DC, Leitch AG. Complexes in serum between alkaline phosphatase and immunoglobulin: immunological and clinical aspects. Clin Chim Acta 1981;111:257-65.
8. Maekawa M, Sudo K, Kanno T. A case of rheumatoid arthritis with various enzyme-immunoglobulin complexes. Clin Chim Acta 1986;157:45-53.
9. Turkcapar N, Ozyuncu N, Idilman R, Ensari A, Soylu K, Ozden A. Macroamylasemia in a patient with selective IgA deficiency and antiphospholipid antibodies. Turk J Gastroenterol 2006;17(2):140-3.
10. Pesce MA. The CK and LD macroenzymes. Lab Management 1984; 22: 29-41.
11. Triester SL, Douglas DD. Development of macro-aspartate aminotransferase in a patient undergoing specific allergen injection immunotherapy. Am J Gastroenterol 2005;100:243-5.
12. Thomas L. Labor und Diagnose. TH Books Verlagsgesellschaft mbH, Frankfurt/Main 2000. p 1583.
13. Kanno T, Sudo K. Properties of amylase-linked immunoglobulins. Clin Chim Acta 1977;76:67-77.
14. Moriyama T, Takebe T, Nobuoka M, Makino M. Characterisation of amylase linked immunoglobulin G to distinguish human salivary and pancreatic iso-amylases. Clin Chim Acta 1988;174:25-34.
15. Klonoff DC. Macroamylasemia and other immunoglobulin-complexed enzyme disorders. West J Med 1980;133:392-407.
16. Briani C, Taninnotto M, Forni M, Bura P. Macroenzymes: too often overlooked. J Hepatol 2003;38:119.
17. Zaman Z, VanOrshoven A, Marien G, Fevery J, Blanckaert N. Simultaneous macroamylasemia and macrolipasemia. Clin Chem 1994;40(6):939-42.
18. Goto H, Wakui H, Komatsuda A, Imai H, Miura AB, Fujita K. Simultaneous macroamylasemia and macrolipasemia in patient with systemic lupus erythematosus in remission. Intern Med 2000;39:1115-8.
19. Konttinen A, Murkos J, Ojala K, Salaspuro M, Somer H, Rasanen J. A new cause of increased serum aspartate aminotransferase activity. Clin Chim Acta 1978;84:145-7.
20. Mofort-Gouraud M, Hamza A, Nacer K, Barjonnet G, Tranie V, Devanaly M, et al. Hypertransaminasemia in adolescents. Arch Pediatr 1999;6(11):1191-2.
21. Rocco O, Carbone A, Lirussi F. Macro-aspartate aminotransferase (macro-AST). A 12-year follow-up study in a young female. Eur J Gastroenterol Hepatol 2003;15(12):1371-3.
22. Liu ZJ, Zhang Y, Zhang XB, Yang X. Observation and identification of lactate dehydrogenase anomaly in postburn patient. Postgrad Med J 2004;80:481-3.
23. Tozawa T. Enzyme-linked immunoglobulins and their clinical significance. Electrophoresis 1989;10:640-4.
24. Otsu N, Hirata M, Miyazawa K, Tuboi S. Abnormal lactate dehydrogenase isoenzyme in serum and tumor tissue of a patient with neuroblastoma. Clin Chem 1985;31:18-20.
25. Perry C, Peretz H, Ben Tal O, Eldor A. Highly elevated lactate dehydrogenase level in a healthy individual: a case of macro-LDH. Am J Hematol 1997;55:39-40.
26. Ishikawa J, Fujita K, Kanno T, Maekawa M. Lactate dehydrogenase (LD) extra isoenzyme electrophoretic band between LD1 and LD2 caused by complex with alfa1-lipoprotein. Clin Chem Lab Med 2004;42(1):102-4.
27. Wright SA, Liggett NW. Elevation of creatine kinase as a marker of malignancy. Irish Med J 2003;96 (7):217.
28. Galarraga B, Sinclair D, Fahie-Wilson MN, McCrae FC, Hull RG, Ledingham JM. A rare but important cause for a raised serum creatine kinase concentration: two case reports and a literature review. Rheumatology 2003;42:186-8.
29. Stein W, Bohner J, Renn W, Maulbetsch R. Macro creatine kinase type 2: results of a prospective study in hospitalised patients. Clin Chem 1985;31:1959-64.
30. Durr HK, Bode C, Krupinski R, Bode JC. A comparison between naturally occurring macroamylasemia and macroamylasemia induced by hydroxyethyl starch. Eur J Clin Invest 1978;8:189-91.
31. Taes YE, Louagie H, Yvergneaux JP, DeBuyzere ML, DePuydt H, Delanghe JR, et al. Prolonged hyperamylasemia attributable to a novel type of macrolipase. Clin Chem 2000;46:2008-13.
32. De Broe ME, Borgers M, Wieme RJ. The separation and characterisation of liver plasma membrane fragments circulating in the blood of patients with cholestasis. Clin Chim Acta 1975;59:369-72.
33. Serdar MA, Tokgoz S, Metinuyrt G, Tapan S, Erinic K, Hasima A, et al. Effect of macro-creatine kinase and increased creatine kinase BB on the rapid diagnosis of patients with suspected acute myocardial infarction in the emergency department. Mil Med 2005;170(8):648-52.
34. Wenham PR, Horn DB, Smith AF. Multiple forms of gamma-glutamyltransferase: a clinical study. Clin Chem 1985;31:569-73.
35. Turecky L, Kupcova V, Laktiš K, Uhlikova E, Szantova M. Gamma-glutamyltransferase isoenzymes in differential diagnosis of chronic liver diseases. Bratisl Lek Listy 1997;98:137-40.
36. Lee KN, Csako G, Bernhardt P, Elin RJ. Relevance of macro creatine kinase type 1 and 2 isoenzymes to laboratory and clinical data. Clin Chem 1994;40:1278-83.
37. Thomas L, Stein W. Diagnostic enzymology. In: Thomas L, ed. Clinical laboratory diagnostics. Use and assessment of clinical laboratory results. Frankfurt/Main: TH-Books Verlagsgesellschaft mbH;1998. p. 29-51.