Introduction
Preeclampsia is a pregnancy-specific disorder clinically characterized by hypertension (blood pressure ≥ 140/90 mm Hg) and proteinuria (≥ 300 mg in a 24-hour urine). It affects approximately 5% of pregnancies and remains a leading cause of both maternal and fetal morbidity and mortality worldwide (1). The etiology and pathogenesis of this disorder remain elusive, resulting in a failure to develop specific preventive and treatment options. The clinical spectrum of this disease covers a wide range of presentations, from mild forms of hypertension and proteinuria, to severe forms such as eclampsia, the convulsive form, and HELLP syndrome. The acronym HELLP (for Hemolysis, Elevated Liver enzymes, and Low Platelet count) was coined in 1982 (2), and refers to the specific laboratory abnormalities that are characteristic of this form of preeclampsia. It is noteworthy that hemolysis and low platelet count have been recognized as complications of severe hypertensive disease in pregnancy for more than 100 years. HELLP affects approximately ten percent of all pregnancies with preeclampsia/eclampsia. In 70% of patients, HELLP syndrome develops antepartum, in the second or third trimester of pregnancy, and in up to 30% it develops postpartum. It is commonly heralded by neurological symptoms and signs, epigastric or right upper quadrant pain in 80-90%, a systolic blood pressure > 160 mm Hg or a diastolic blood pressure > 110 mm Hg in 50%, proteinuria in 94%, and acute renal failure in 7.4% of patients (3,4,5). Medical treatment typically consists of magnesium sulfate seizure prophylaxis (6) and hypertension treatment for a blood pressure > 150/100 mm Hg.
Studies of maternal outcomes in pregnancies complicated by HELLP syndrome have demonstrated increased risks for disseminated intravascular coagulation, placental abruption, acute renal failure, pulmonary edema, subcapsular liver hematoma, and retinal detachment (7,8). Consequently, the presence of HELLP syndrome since its initial description has been considered a major indication for immediate delivery. Therefore, little is known about the natural course and pregnancy outcomes in untreated patients, i.e., those treated conservatively, despite the presence of clinical signs and laboratory abnormalities that are indicative of this syndrome.
The objectives of this study were i) to study the natural course of HELLP syndrome in patients who were treated for preeclampsia prior to 1982, before its recognition as a distinct syndrome, and who, in retrospect, met the diagnostic criteria, and ii) to study whether an increased awareness of HELLP syndrome after 1986 resulted in more timely diagnosis, earlier treatment, and hence improved maternal and fetal outcomes.
Materials and methods
Patient selection
The protocol was approved by the Institutional Review Board and all subjects had consented to the use of their records for research. Relevant clinical data were abstracted from the records of patients with preeclampsia treated at Mayo Clinic before and after 1982.
A retrospective diagnosis of HELLP in 11 of 146 patients treated for preeclampsia/eclampsia prior to 1982 was confirmed by the presence of microangiopathic hemolytic anemia, elevated liver enzymes, and thrombocytopenia, according to previously accepted diagnostic criteria (4,9) as follows:
Evidence of intravascular hemolysis:
- Decreasing hemoglobin with at least one of the following:
- Abnormal peripheral blood smear (schistocytes)
- Elevated lactate dehydrogenase activity (LD), greater than 600 U/L
- Elevated total bilirubin, equal or greater than 1.2 mg/dL
Elevated liver enzymes:
- Aspartate aminotransferase (AST), greater than 70 U/L
- LD, greater than 600 U/L
Low platelet count:
- Platelet count lower than 100 x 109/L
Based on the same set of criteria, the diagnosis of HELLP was verified in a randomly selected group of 24 women treated for HELLP syndrome between the years 1986-1994. The year 1986 was chosen as the lower range for the control group to allow several years for HELLP to become a universally recognized syndrome requiring urgent attention. Of note, all the patients had a coexisting diagnosis of preeclampsia, as defined by a blood pressure (≥ 140/90 mm Hg) and proteinuria (≥ 300 mg in a 24-hour urine) (1). Perinatal mortality included deaths occurring during late pregnancy (at 22 completed weeks of gestation and over), during childbirth, and up to seven completed days of life.
Data collection
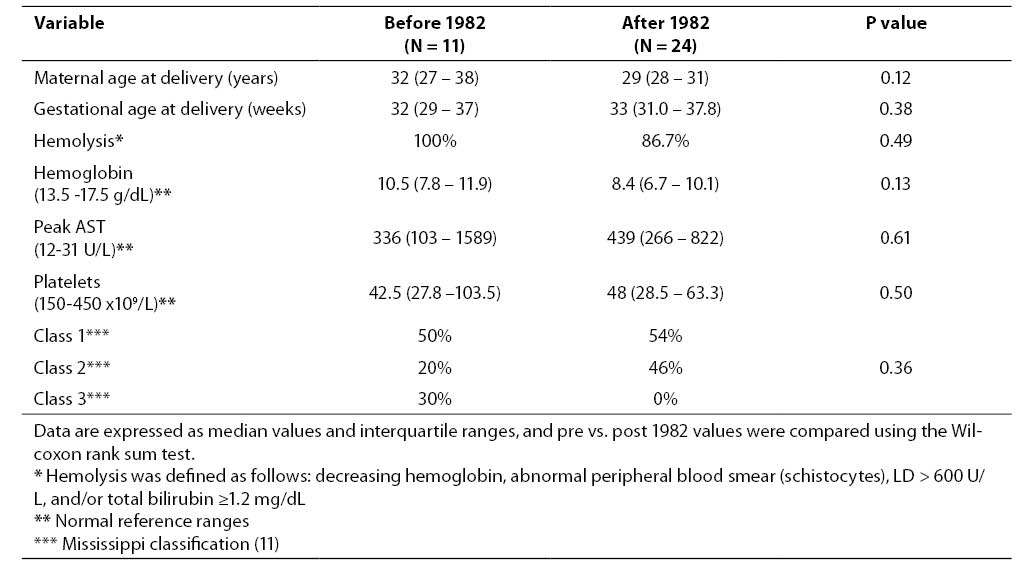
The following data were collected: maternal age, gestational age, mode of delivery, maternal mortality, intrauterine fetal death, maternal complications (pulmonary edema, pleural effusion, pericardial effusion, intracerebral hemorrhage, seizures, abruptio placentae, hepatic rupture, and retinal detachment), time from diagnosis to delivery, seizure prophylaxis with MgSO4, and laboratory results. Acute renal failure was defined as an increase in serum creatinine of 1.5 times, or a decrease in the glomerular filtration rate by more than 25%, compared to baseline (10). There was no difference between groups with respect to disease severity as determined by the Mississippi classification, which uses the platelet count to define HELLP syndrome as severe (≤ 50 x 109/L), moderate (50 - 100 x 109/L), or mild (> 100 x 109/L) (11). In addition, maternal demographics and diagnostic laboratory values were similar between the groups (Table 1).
Table 1. Maternal characteristics and diagnostic laboratory data of HELLP syndrome.
Statistical analysis
Descriptive statistics are reported for quantitative traits as medians and interquartile ranges, and for categorical traits as percentages. Statistical procedures included the Wilcoxon rank sum test for continuous variables, Fisher’s exact test for categorical variables, exact inference for ordered contingency tables, and the Kaplan-Meier method was utilized for time to event analysis. A P value of < 0.05 was pre-specified as being statistically significant.
Results
Major outcomes
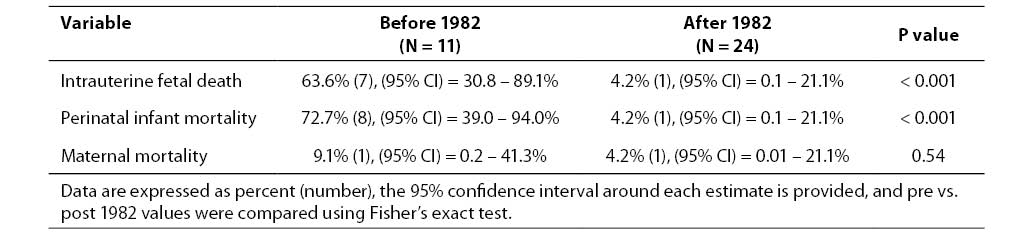
In our study population, the perinatal death rate was significantly higher prior to 1982. In that group, 8 of 11 infants (73%) were either stillborn (N = 7), or died hours after birth (N = 1). In contrast, only 1 of 24 (4%) infants born to mothers after 1986 was stillborn. This difference was statistically significant (P < 0.001). There was no difference in the maternal death rate between groups, with 1 of 11 mothers dying prior to 1982 and 1 of 24 dying after 1986 (P = 0.5), (Table 2).
Table 2. Comparisons of major outcomes between the two groups.
Maternal complications
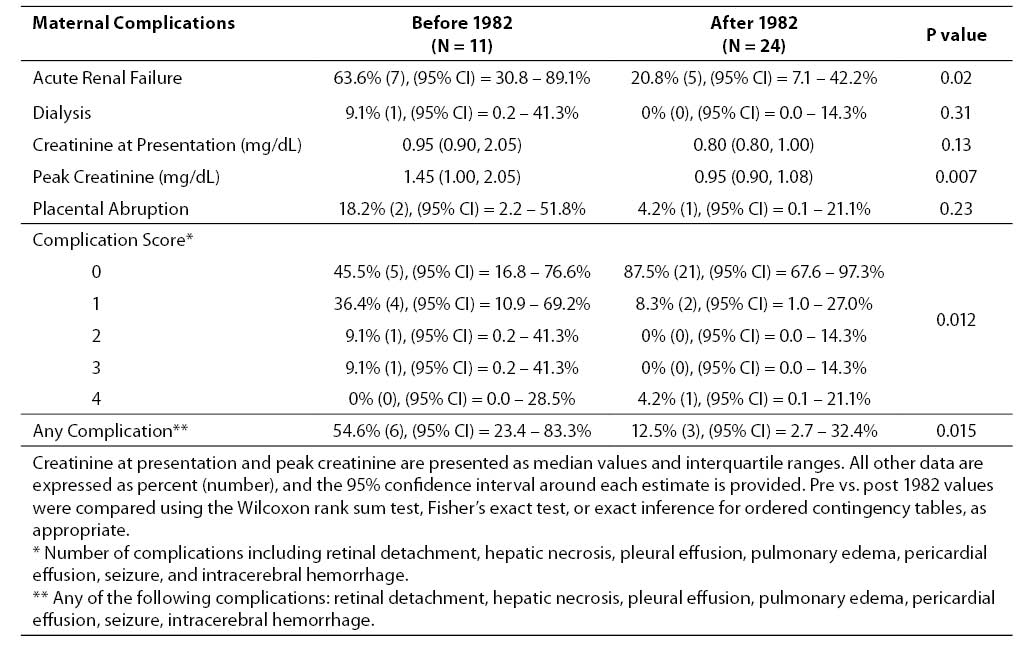
Prior to 1982, significantly more women with HELLP syndrome developed renal failure (63.6% vs. 20.8% P = 0.02). The median peak creatinine was higher in these patients as well (1.45 mg/dL vs. 0.95 mg/dL, P = 0.007). There was no difference in the number of women requiring dialysis (1 of 11 vs. 0 of 24, P = 0.3).
In addition to renal failure, we also calculated a “complication score” that was the sum of all other complications recorded in the medical record. Complications included in this score are pulmonary edema, pleural effusion, pericardial effusion, retinal detachment, hepatic necrosis, intracerebral bleeding, and seizure. We found that women treated prior to 1982 had a significantly higher number of total complications (P = 0.012), and that the total number of women with any complications was significantly higher in this group (54.6% vs. 12.5% P = 0.015), (Table 3).
Table 3. Comparisons of maternal complications between the two groups.
Practice changes
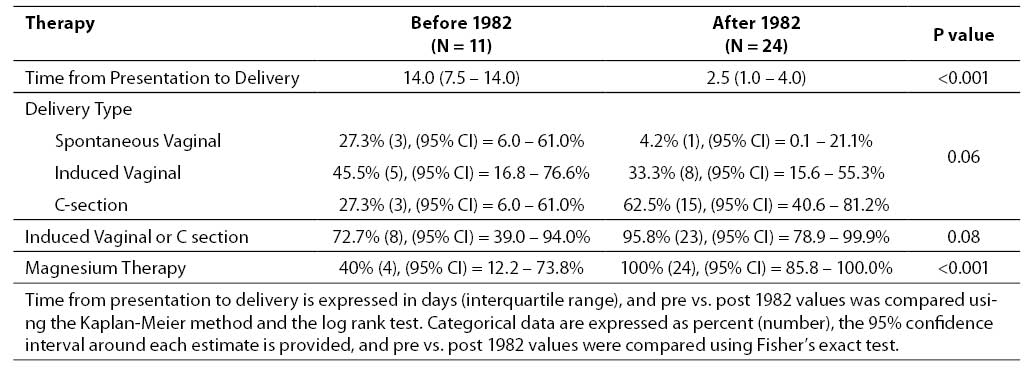
After its recognition, the treatment of women with HELLP syndrome changed. Most importantly, there was a significant decrease in the length of time between presentation and either live birth or delivery of a stillborn infant (14 days pre-1982 vs. 2.5 days post-1982, P < 0.001). After 1982, there was a marginally significant trend towards more women being delivered by induction or Caesarian section versus spontaneous delivery (P = 0.08). More women were treated with magnesium therapy for seizure prophylaxis as well, although this may reflect practice changes over time independent of the recognition of HELLP as a syndrome (Table 4).
Table 4. Comparisons of practice differences (time to and mode of delivery, magnesium sulfate prophylaxis) between the two groups.
Discussion
Our study provides evidence that the definition of HELLP as a distinct clinical syndrome has led to improved outcomes of pregnancy, likely due to a more timely diagnosis, leading to an earlier termination of pregnancy. Pregnancies of women diagnosed with HELLP syndrome after 1982 were delivered a median of 2.5 days after presentation, which is in keeping with current practices that call for delivery of patients with strictly defined HELLP (i.e., presence of all 3 diagnostic criteria) in less than or equal to 48 hours from diagnosis. In contrast, women prior to 1982 were delivered a median of 14 days after conservative management. In addition, our study suggests that prior to 1982, pregnancies in women who presented with laboratory abnormalities that, in retrospect, were diagnostic of HELLP syndrome, were associated with significant maternal morbidity, mortality, and perinatal death rate.
Similar to previous studies (8), we report that women with HELLP syndrome are at risk for complications, such as pulmonary edema, pleural effusion, pericardial effusion, retinal detachment, hepatic necrosis, intracerebral bleeding and seizures, with women treated prior to 1982 being at a higher risk for these complications compared to those treated after 1982. In addition, women treated prior to 1982 had more serious renal involvement, both with respect to the incidence and severity of acute renal failure. The most striking difference was observed with respect to fetal outcomes: the majority of pregnancies (73%) before 1982 ended with either intrauterine fetal death or infant loss, while only one intrauterine fetal death was recorded after 1982. More aggressive treatment after 1982 is evidenced further by a trend towards more pregnancies being promptly delivered either by labor induction or Cesarean section. Finally, more women after 1982 were treated with magnesium sulfate, based on the evidence that supported the use of magnesium sulfate for seizure prophylaxis (12). As the diagnosis of HELLP is largely based on laboratory abnormalities, our study underscores the role of timely laboratory testing and the expertise of laboratory professionals in the diagnosis and subsequent monitoring of these patients.
Despite a proactive approach and immediate delivery, women treated after 1982 still experienced significant complications, although at much lower rates than those treated more conservatively before 1982. These results extend previous reports of adverse maternal-perinatal outcomes in women with HELLP syndrome (3,13-16), and emphasize the need for further research in this field that may result in more effective diagnostic and treatment strategies.
The limitations of our study relate to its retrospective design. Conceivably, for patients before 1982, laboratory parameters that facilitated the retrospective diagnosis of HELLP might have been available only for those who were very ill, thus preselecting for particularly bad pregnancy outcomes, and resulting in the high perinatal mortality that we report (73%). However, this percentage is not significantly different from the upper range of perinatal mortality that has been reported as high as 60%, even since the recognition of HELLP in 1982 (4). On the other hand, we reported a relatively low perinatal mortality after 1982, 4.2%. It is likely a reflection of not only timely diagnosis and treatment of HELLP, but also the availability of advanced perinatal care in a tertiary care center. Despite these limitations, our data clearly indicate improved maternal and fetal outcomes after 1982, when the clinical recognition of HELLP syndrome usually led to immediate delivery.
Some authors have suggested intravenous dexamethasone as treatment to raise platelet counts and improve liver function test abnormalities (17). This approach has resulted in a modest delay in delivery (č25 hours) and is not widely accepted. In contrast to preeclampsia, which typically occurs during the first pregnancy, HELLP syndrome tends to occur in multiparous women. The development of HELLP may be heralded by epigastric pain and clinical signs and symptoms of severe preeclampsia, although it may develop in patients without significant hypertension and/or proteinuria. In up to 30% of patients, symptoms and signs of HELLP develop only in the postpartum period (4). Making a final diagnosis depends upon obtaining laboratory reports and looking for HELLP-defining characteristics by obtaining a complete blood count with peripheral smear, LD, AST and ALT. Once the diagnosis of HELLP is established, the course of the disease should be monitored by following hematocrit, platelet count, LD, AST and ALT activity every six hours. The platelet count is important not only for diagnosis, but for assessing the severity of the disease as well (11). Of note, hepatic rupture, one of the most severe complications of the HELLP syndrome, typically occurs in patients with established thrombocytopenia and extremely elevated liver enzymes.
The natural history of HELLP syndrome with respect to laboratory abnormalities during the peripartum period was studied by Martin et al. in 158 HELLP patients who were managed at a single tertiary referral center (18). They reported that the platelet count tends to decrease 24-48 hours after delivery, while LD activity typically peaks during the same time period. In patients without complications, an upward trend in platelet count and downward trend in LD activity by the fourth post partum day signal recovery. Therefore, serial measurements of LD activity and platelet count are the two best markers of the course of the disease. As a LD activity increase may reflect both hepatic damage and hemolysis, the analysis of LD isoenzymes may provide further information: the elevations of LD 1 and LD 2 reflect the severity of hemolysis, while liver damage contributes to elevations in LD 5. A rise in AST and ALT activity reflects liver damage and typically occurs before biliary obstruction develops, which is reflected by elevations in bilirubin and alkaline phosphatase. Additional tests that may help in diagnosis and monitoring of patients with HELLP syndrome include haptoglobin, magnesium, and glucose levels. A decrease in the haptoglobin level is a sensitive marker of hemolysis, low glucose levels may be a marker of severe liver damage, while serial magnesium levels are followed to assure adequate levels are achieved (č6mg/dL) to prevent seizures, on one hand, while preventing an accumulation of toxic levels on the other, especially in patients with underlying renal insufficiency. In addition, all patients should undergo laboratory testing that is performed for patients with either suspected or confirmed preeclampsia, including urinalysis with either a random or 24-hour protein determination, urea, creatinine, and serum electrolytes. Clinically, blood pressure and urine output should be followed hourly. Finally, further testing of coagulation parameters such as fibrinogen, fibrin split products or D-dimer, and prothrombin and partial thromboplastin times, may facilitate the diagnosis of disseminated intravascular coagulation, a condition that occurs in 15-20% of HELLP pregnancies (8,19).
The full laboratory profile of HELLP may be absent in up to 50% of patients. (19). In cases of partial HELLP, when one or two, but not all three features of HELLP are present, the rate of severe complications does not appear to be as high as in cases where all criteria of HELLP are met. Therefore, appropriate and timely testing is not only important for the initial diagnosis, but also in determining overall prognosis.
The reasons why some women with preeclampsia develop HELLP, while others do not, remain poorly understood (20). However, recent research has indicated that women with HELLP syndrome demonstrate elevated levels of a soluble transforming growth factor β co-receptor, endoglin, (21) that may act in concert with soluble fms-like tyrosine kinase 1 (sFlt-1), which has been shown previously to be elevated in sera of patients with preeclampsia (22). sFlt-1 may contribute to the pathogenesis of preeclampsia by binding and neutralizing vascular endothelial growth factor (VEGF), thus decreasing free VEGF levels that are required for active fetal and placental angiogenesis in pregnancy. However, to date, urine and serum measurements of these factors have not provided clinically reliable screening tools with current techniques.
Conclusion
In summary, we conclude that the recognition of HELLP syndrome as a deceptive and severe form of preeclampsia in 1982 has since resulted in improved maternal and fetal outcomes, mainly due to the widely accepted practice of immediate delivery. As it is manifested as a combination of laboratory abnormalities, including hemolysis, abnormal liver tests, and thrombocytopenia, the role of laboratory professionals in the diagnosis, serial monitoring, and therapy of these patients increasingly is recognized (9).
Acknowledgments
We thank Ms. Heather J. Wiste and Christina M. Wood for assistance with statistical analyses.
References
1. Anonymous. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183(1):S1-S22.
2. Weinstein L. Syndrome of hemolysis, elevated liver enzymes, and low platelet count: a severe consequence of hypertension in pregnancy. Am J Obstet Gynecol. 1982;142(2):159-67.
3. Sibai BM, Taslimi MM, el-Nazer A, Amon E, Mabie BC, Ryan GM. Maternal-perinatal outcome associated with the syndrome of hemolysis, elevated liver enzymes, and low platelets in severe preeclampsia-eclampsia. Am J Obstet Gynecol. 1986;155(3):501-9.
4. Sibai BM. The HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets): much ado about nothing? Am J Obstet Gynecol. 1990;162(2):311-6.
5. Sibai BM, Villar MA, Mabie BC. Acute renal failure in hypertensive disorders of pregnancy. Pregnancy outcome and remote prognosis in thirty-one consecutive cases. Am J Obstet Gynecol. 1990;162(3):777-83.
6. Altman D, Carroli G, Duley L, Farrell B, Moodly J, Neilson J, et al. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. The Lancet. 2002;359(9321):1877-90.
7. Egerman RS, Sibai BM. HELLP syndrome. Clin Obstet Gynecol 1999;42(2):381-9.
8. Sibai BM, Ramadan MK, Usta I, Salama M, Mercer BM, Friedman SA. Maternal morbidity and mortality in 442 pregnancies with hemolysis, elevated liver enzymes, and low platelets (HELLP syndrome). Am J Obstet Gynecol. 1993;169(4):1000-6.
9. Jones SL. HELLP! A cry for laboratory assistance: a comprehensive review of the HELLP syndrome highlighting the role of the laboratory. Hematopathol Mol Hematol. 1998;11(3-4):147-71.
10. Bellomo R, Ronco C, Kellum J, Mehta R, Palevsky P, workgroup tA. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Critical Care. 2004;8(4):R204 - R212.
11. Martin JN, Jr., Blake PG, Lowry SL, Perry KG, Jr., Files JC, Morrison JC. Pregnancy complicated by preeclampsia-eclampsia with the syndrome of hemolysis, elevated liver enzymes, and low platelet count: how rapid is postpartum recovery? Obstet Gynecol. 1990;76(5 Pt 1):737-41.
12. Sibai BM. Magnesium sulfate is the ideal anticonvulsant in preeclampsia-eclampsia. Am J Obstet Gynecol. 1990;162(5):1141-5.
13. Moodley J. Maternal deaths associated with hypertensive disorders of pregnancy: a population-based study. Hypertens Pregnancy. 2004;23(3):247-56.
14. Gul A, Aslan H, Cebeci A, Polat I, Ulusoy S, Ceylan Y. Maternal and fetal outcomes in HELLP syndrome complicated with acute renal failure. Ren Fail. 2004;26(5):557-62.
15. Deruelle P, Coudoux E, Ego A, Houfflin-Debarge V, Codaccioni X, Subtil D. Risk factors for post-partum complications occurring after preeclampsia and HELLP syndrome. A study in 453 consecutive pregnancies. Eur J Obstet Gynecol Reprod Biol. 2006;125(1):59-65.
16. Araujo AC, Leao MD, Nobrega MH, Bezerra PF, Pereira FV, Dantas EM, et al. Characteristics and treatment of hepatic rupture caused by HELLP syndrome. Am J Obstet Gynecol. 2006;195(1):129-33.
17. Magann EF, Bass D, Chauhan SP, Sullivan DL, Martin RW, Martin JN, Jr. Antepartum corticosteroids: disease stabilization in patients with the syndrome of hemolysis, elevated liver enzymes, and low platelets (HELLP). Am J Obstet Gynecol. 1994;171(4):1148-53.
18. Martin JN, Jr., Blake PG, Perry KG, Jr., McCaul JF, Hess LW, Martin RW. The natural history of HELLP syndrome: patterns of disease progression and regression. Am J Obstet Gynecol. 1991;164(6 Pt 1):1500-13.
19. Audibert F, Friedman SA, Frangieh AY, Sibai BM. Clinical utility of strict diagnostic criteria for the HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome. Am J Obstet Gynecol. 1996;175(2):460-4.
20. August P. Preeclampsia: New Thoughts on an Ancient Problem. J Clin Hypertens. 2000;2:115-23.
21. Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355(10):992-1005.
22. Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350(7):672-83.