Introduction
Acute inflammation is a crucial initial phase in the wound healing process that leads to structural and functional repair of injured tissue. Prompt initiation of the inflammatory cascade occurs primarily through activated blood monocytes and tissue macrophages at the wound site, and the released inflammatory mediators such as IL-1 and IL-6, also causing systemic changes (1). One of the most intensively studied systemic responses to an acute inflammatory stimulus is the rapid increase in serum concentrations of acute phase proteins (APPs) (2,3). Serum amyloid A protein (SAA) is a low molecular weight APP, which is produced primarily by the liver in response to pro-inflammatory cytokines. The SAA level in blood exhibits the most intense and rapid increase among all APPs and therefore is a sensitive indicator of inflammation and valuable in monitoring efficacy of antimicrobial and anti-inflammatory therapy (4-6). Interferons belong to the network of cytokines that are involved in the control of cellular function and they play an important role in macrophage activation, especially interferon-gamma (IFN-γ), an important activator of the immune response and modulator of wound healing (7,8). IFN-g has been implicated in the homeostasis of epidermal keratinocyte proliferation (9), and systemic administration of IFN-g upon injury decreased wound collagen deposition and clearly reduced the initial inflammatory response (10).
Diabetes is well known to be associated with delayed healing of wounds and genetically diabetic (db/db) mice are useful as an animal model for this condition, since wound healing in these animals is markedly delayed as compared to non-diabetic C57Bl/6 littermates (11,12). Healing impairment is characterized by delayed cellular infiltration and granulation tissue formation, reduced angiogenesis, decreased collagen and its organization (13,14). In db/db mice, development of diabetes is coupled with the absence of the functional isoform of the leptin receptor (15,16). Leptin, a product of the ob gene, is synthesized by adipose tissue and its level correlates with the amount of body fat (17). It is involved in energy balance regulation but also in the acute phase response to tissue injury and systemic inflammation, probably because the leptin transmembrane receptor has structural similarities to the IL-6-like cytokine family (18,19).
In this study, we investigated changes in the systemic inflammatory response, as reflected by SAA, IFN-g and neutrophil and lymphocyte differential counts in peripheral blood during cutaneous wound repair in db/db mice and their littermate controls to determine whether the systemic inflammatory response is also delayed in db/db mice. In this way, we hoped to be able to determine whether systemic inflammatory parameters could be used as biomarkers for local wound healing.
Materials and methods
Animals
Twenty genetically diabetic female C57BL/KsJ db+/db+ mice and twenty female C57Bl/6 control mice, all 6 weeks old, were obtained from Charles River Laboratories, Belgium. Animals were allowed to acclimatize for 10 days, marked and individually housed for the experiments. Mice were kept under standard laboratory conditions. Food and water were provided ad libitum.
All procedures on animals were performed in accordance with the (a) EEC Council Directive 86/609 of November 24, 1986 on the approximation of laws, regulations and administrative provisions of the Member States regarding the protection of animals used for experimental and other scientific purposes; and (b) Statute of Republic Croatia, Animal Welfare Law, Official Gazette 081-99-266/1 of February 9, 1999.
Experimental procedure
Diabetic db/db mice and non-diabetic C57BL/6 mice were divided into 4 groups of 5 animals. On day -1, mice were anesthetized by inhalation of isoflurane (Forane, isoflurane, inhalation anesthetic, Abbott Laboratories, England) and 5% oxygen, delivered in an anesthesia induction chamber (Stoelting Co., USA). Gas scavenging was provided using the Fluovac 240V system (International Market Supply, England). Subsequently, anesthesia was maintained, using a mask (Stoelting Co., USA), with isoflurane and 4% oxygen, the pedal reflex response being checked at intervals. In the fully anesthetized animal, the interscapular region was surgically close-shaved (Contura cordless clipper, International Market Supply, England), brushing off excess loose hair. Twenty-four hours later (day 0), animals were anesthetized as on day -1 and the shaved region was disinfected (Pursept-A, Merz Hygiene, Germany). Utilizing strictly aseptic procedures, a single full-thickness excisional wound 8 mm in diameter was made midline in the shaved region of each animal (20), with a sterile, disposable biopsy punch (Stiefel Laboratories Ltd., Ireland), exposing the underlying muscular fasciae. Animals were returned to their cages with some form of distractive enrichment like food pellet, and allowed to recover from the anesthesia. On days 3, 6, 9 and 13 five db/db and five C57Bl/6 mice were anesthetized as before and exsanguinated by puncturing the jugular vein and common carotid artery into Becton Dickinson EDTA containing microtainers.
Analysis of wound closure
Serial standard 2D photographs of each wound were made with an Olympus C-2040 Zoom digital camera (Olympus Optical Co., Ltd., Japan) immediately after wounding (day 0) and on days 1, 3, 6 and 9 while animals were under isoflurane anesthesia. Digital images were processed using the Leica QWin Image Processing and Analysis System (Leica Imaging System Ltd., UK), the manual tool bar allowing manual tracing of the wound margins. Wound area was calculated for each image.
Sample preparation and analyses
Aliquots of blood samples were pre-diluted with Sysmex Cell-pack solution at a 1:5 ratio and analyzed on the Sysmex SF 3000 automatic hematology analyzer within 4 h of withdrawal. Afterward, blood was centrifuged at 3500 rpm for 15 min at room temperature. Plasma aliquots were stored frozen at -20 °C until analyzed.
In order to determine the levels of SAA, samples were pre-diluted 1:200. SAA concentrations were determined by commercial ELISA (Phase Serum Amyloid A Assay (Murine), Tridelta, Ireland) research kit (CV < 10%, analytical sensitivity 0.03 μg/mL).
IFN-γ was measured by commercial ELISA (Mouse IFNg Biotrak Assay, Amersham Biosciences, UK) research kit (CV < 10%, sensitivity < 10 pg/mL).
Statistical analysis and evaluation
Statistical calculations for all parameters were performed using GrafPad software. In order to determine the difference between db/db and healthy C57Bl/6 mice, raw data were analyzed by Mann-Whitney test. The difference between time points within the same group was analyzed by non-parametric one-way analysis of variance (ANOVA) using Kruskal-Wallis test with Dunn’s multiple comparison test. The level of significance was set at P < 0.05. All parameters are presented as medians with 95% confidence limits, except for SAA (medians with 1st and 3rd quartiles).
Results
Wound closure
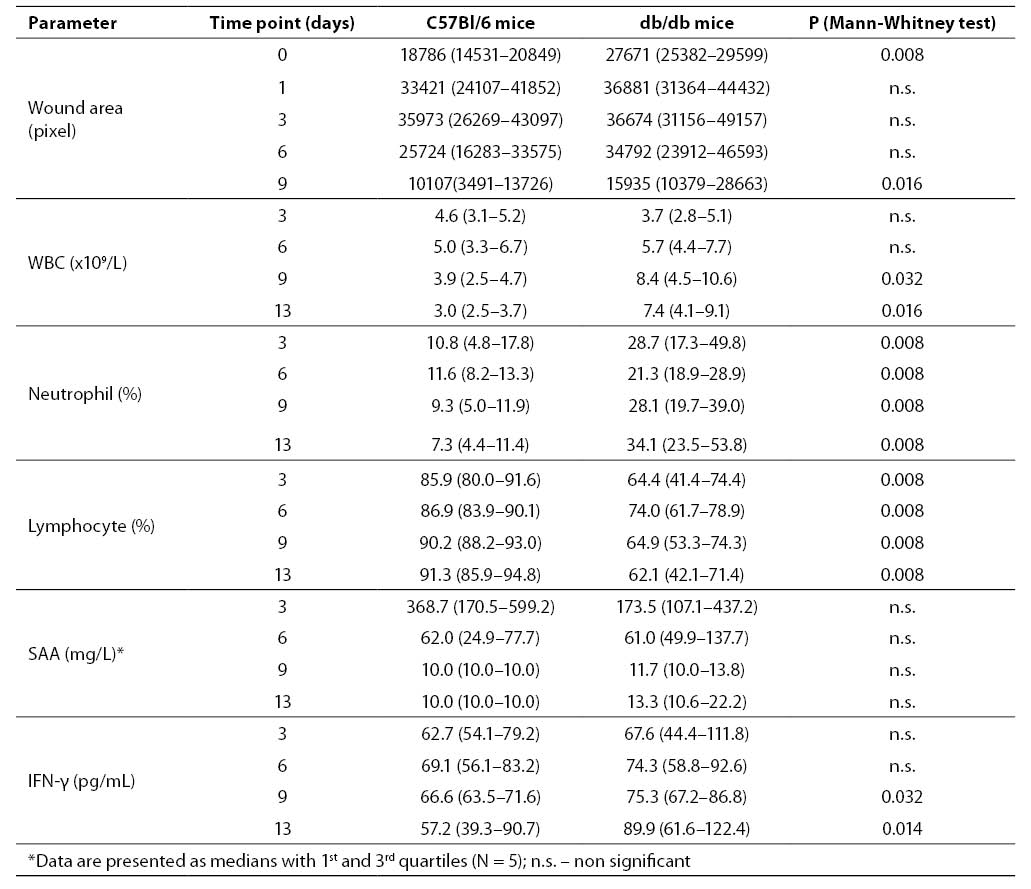
Excision of skin resulted in retraction of the wounds within the first day in all animals and was significantly greater in C57Bl/6 mice (Table 1). On day 0, wound areas in db/db mice were significantly greater than in C57Bl/6 littermates, indicating reduced elasticity in the wound margins of diabetic, obese animals. In db/db mice, wound retraction continued till day 6, while in C57Bl/6 mice at that time wound closure had already started. On day 3, relative wound size in C57Bl/6 was the same as in db/db mice, while on day 9 it was significantly smaller in C57Bl/6 mice than in db/db mice, indicating significantly faster wound closure in non-diabetic C57Bl/6 mice. By day 13, all wounds in C57Bl/6 and 70% of wounds in db/db mice were healed (data not shown).
Hematology parameters
Total white blood cell (WBC) count in blood from C57Bl/6 mice increased and reached peak value on day 6, subsequently falling to day 13, achieving statistical significance (P = 0.037) (Table 1). In db/db mice, total WBC count reached peak value later (day 9) in relation to non-diabetic littermates reaching statistical significance vs. day 3 (P = 0.048), and remained relatively high until the end of the study (day 13). On days 9 and 13, total WBC count was significantly higher in db/db mice than in C57Bl/6 mice (P = 0.032 and P = 0.016, respectively) (Table 1). The WBC reference range for female C57Bl/6 mice, according to Charles River Laboratories, is 8.09 ± 2.29 x 109/L.
Neutrophil differential counts in blood from C57Bl/6 mice decreased gradually throughout the observation period. Neutrophil differential counts in blood of db/db mice on day 3 were almost three-fold those in healthy mice, and this increase was sustained throughout the observation period (Table 1). On day 6, neutrophil percentage of db/db mice decreased, however, it did not reach statistical significance. Lymphocyte differential count in blood from healthy mice showed a slight increase towards the end of the study. In contrast, lymphocyte count in blood from db/db mice increased until day 6, followed by a decrease until the end of the study, however, without reaching statistical significance (Table 1).
SAA and IFN-g
In mice, the SAA reference range is 0.2–40 mg/L (21,22). In wounded C57Bl/6 mice, SAA concentration declined from day 3 to day 6, however, not reaching statistical significance. On days 9 and 13 after the wound incision, SAA levels in all animals were below 40 mg/L, within the normal range (Table 1). In wounded db/db mice, SAA levels were increased in all 5 animals on day 3 in relation to the reference range. Median values (with 1st and 3rd quartiles) were lower than in C57Bl/6 mice at the same time point, yet not achieving statistical significance. As in non-diabetic littermates, SAA concentration in db/db mice returned to the SAA reference range on days 9 and 13 (Table 1).
IFN-γ concentrations in blood from wounded db/db mice tended to be higher in relation to C57Bl/6 mice during the study period, but this difference only reached statistical significance on days 9 and 13 (Table 1).
Table 1. Parameters measured in C57Bl/6 and db/db mice during the study period. Data are presented as medians with 95% confidence limits (N = 5)
Discussion
We have confirmed, using a previously developed, precise and reproducible computerized method for monitoring skin wound closure (23,24), that wound healing is markedly delayed in diabetic db/db mice. We have also extended these observations to show that the systemic inflammatory and acute phase response is delayed and more sustained than in non-diabetic littermates.
It is well known that in diabetic humans and animal models of diabetes, such as the db/db mouse, wound healing is delayed (7,12). From our study, it is clear that the effect of the diabetic disease process has at least two components. On the one hand, the mechanical retraction of the wound margin is impaired in db/db mice in comparison to normal mice. Rodent skin is different to human skin and mechanical retraction is an important initial response to tissue injury in the rodent and not in humans. The impaired wound retraction in db/db mice is probably attributable to the large amount of subcutaneous adipose tissue in these obese animals, but may also be related to changes in the elasticity of the connective tissue. On the other hand, re-epithelialization and deposition of granulation tissue in the wounds of these diabetic mice are also impaired, resulting in a delay in wound closure also seen in our study.
Although extensive studies have been performed on the effects of the leptin receptor deficiency on local changes in healing wounds in db/db mice, little is known about systemic inflammatory changes under these conditions. We found an increased and sustained WBC count that lasted for up to at least 13 days after wounding in diabetic db/db as opposed to normal mice. This was predominantly accounted for by an increase in the circulating neutrophil count. These findings correlate very well with those of a previous study by Wetzler et al. (25) who showed that in full-thickness excision wounds in db/db mice, local inflammation, as measured by IL-1β and TNF-a expression, as well as the infiltration of the tissue by neutrophils and macrophages were markedly enhanced and prolonged in comparison to healthy C57bl/KS mice for at least 13 days. This enhanced local inflammatory response was associated with increased MIP-2 and MCP-2 chemokine expression in the wound tissue. The systemic hematological changes we observed, therefore, seem to reflect closely the local cell infiltration occurring at the wound site in db/db mice. Moreover, lymphocyte development in the thymus of db/db mice is impaired (26), which may have contributed to the reduced lymphocyte differential cell count observed in our study. The sustained inflammatory response in db/db mice may contribute to the delay in wound healing by delaying the change from inflammation to connective tissue deposition.
Acute phase proteins are frequently used as markers of the systemic response to acute or sub-acute inflammation. Measurement of SAA concentrations (SAA is the most important acute phase protein in mice) in our study did not significantly reflect the changes occurring at the wound site since SAA increases in response to injury early and has a short lifetime in circulation. This was a limitation to our study since the first time point was on day 3. However, although there was no statistical significance, SAA levels were lower on day 3 in db/db than in normal mice. These findings correlate with those of other authors, showing that after the intra-articular injection of zymosan A into the joints of ob/ob (leptin-deficient rather than leptin receptor deficient) mice, early circulating levels of SAA (27) are decreased in association with the enhanced arthritic response in these diabetic animals (28).
IFN-γ is very important in the process of wound closure. It is barely detectable in healing excisional wounds in db/db mice, possibly due to high levels of the suppressing cytokine TGF-β1 (29). This probably accounts for the lack of difference in circulating IFN-γ concentrations between db/db and C57bl/6 mice on days 3 and 6 in our study. However, the circulating levels of this cytokine significantly increased in db/db mice only on days 9 and 13, possibly reflecting the sustained inflammatory response at the wound site and/or systemic inflammatory stimulation of other organs. Indeed, in response to lipopolysaccharide, the generation of pro-inflammatory cytokines by heart and aorta of db/db mice has been reported to be 13- to 30-fold that in normal mice (29). The late rise in IFN-γ may help induce a change in the wound site from inflammation to tissue repair, in view of the mutually antagonistic effects of IFN-γ (30) and TGF-β (10,31,32).
While the concentrations of different tissue to systemic IFN-γ concentrations in wounded animals may limit the use of this cytokine as a marker of local wound response, SAA measurement needs further investigation to find out whether it can be used as a more appropriate marker of local inflammation during the wound healing process. We also suggest using earlier time points to see whether it may predict the dynamics of local wound healing.
In conclusion, the local tissue regeneration process in mice after local skin injury causes systemic changes in peripheral blood. Determination of neither SAA nor IFN-γ concentrations could be used to monitor wound healing dynamics at these time points.
Acknowledgments
We would like to thank Slavica Skender and Vedran Vrban for their excellent technical assistance.
References
1. Holzheimer RG, Steinmetz WG. Local and systemic concentration of pro- and anti-inflammatory cytokines in human wounds. Eur J Med Res 2000;5:347-55.
2. Uhlar CM, Whitehead AS. Serum amyloid A, the major vertebrate acute-phase reactant. Eur J Biochem 1999;265:501-23.
3. Mambule C, Ando Y, Anan I, Holmgren G, Sandgren O, Stigbrandt T et al. Enhancement of AA-amyloid formation in mice by transthyretin amyloid fragments and polyethylene glycol. Biochim Biophys Acta 2000;1474:331-6.
4. Grehan S, Herbert J, Whitehead AS. Down-regulation of the major circulating precursors of proteins deposited in secondary amyloidosis by a recombinant mouse interleukin-1 receptor antagonist. Eur J Immunol 1997;27:2593-9.
5. Lindhorst E, Young D, Bagshaw W, Hyland M, Kisilevsky R. Acute inflammation, acute phase serum amyloid A and cholesterol metabolism in the mouse. Biochim Biophys Acta 1997;1339:143-54.
6. Yamada T. Serum amyloid A (SAA): a concise review of biology, assay methods and clinical usefulness. Clin Chem Lab Med 1999;37:381-8.
7. De Maeyer E, De Maeyer-Guignard J. Interferons. In: Thomson A, ed. The Cytokine Handbook. San Diego: Academic Press; 1998. p. 491-516.
8. Miles RH, Paxton TP, Zacheis D, Dries DJ, Gamelli RL. Systemic administration of interferon-gamma impairs wound healing. J Surg Res 1994;56:288-94.
9. Fransson J, Shen Q, Scheynius A, Hammar H. The effect of IFN-gamma on healthy and psoriatic keratinocytes in a skin equivalent model is influenced by source of the keratinocytes and by their interaction with fibroblasts. Arch Dermatol Res 1996;289:14-20.
10. Laato M, Heino J, Gerdin B, Kahari VM, Niinikoski J. Interferon-gamma-induced inhibition of wound healing in vivo and in vitro. Ann Chir Gynaecol 2001;90 (Suppl 215):19-23.
11. Senter LH, Legrand EK, Laemmerhirt KE, Kiorpes T. Assessment of full-thickness wounds in the genetically diabetic mouse for suitability as a wound healing model. Wound Repair Regen 1995;3:351-8.
12. Davidson JM. Animal models for wound healing repair. Arch Dermatol Res 1998;290 (Suppl):1-11.
13. Goodson VH, Hung TK. Studies of wound healing in experimental diabetes mellitus. J Surg Res 1977;22:221-7.
14. Greenhalgh DG, Sprugel KH, Murray MJ, Ross R. PDGF and FGF stimulate wound healing in the genetically diabetic mouse. Am J Pathol 1990;136:1235-46.
15. Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 1996; 84:491-5.
16. Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, le JI et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature 1996;379:632-5.
17. Ghilardi N, Ziegler S, Wiestner A, Stoffel R, Heini MH, Skoda RC. Defective STAT signaling by the leptin receptor in diabetic mice. Proc Natl Acad Sci USA 1996;93:6231-5.
18. Lofreda S, Yang SQ, Lin HZ, Karp CL, Brengman ML, Wang DJ et al. Leptin regulates proinflammatory immune responses. FASEB J 1998; 12:57-65.
19. Fruhbeck G. Intracellular signalling pathways activated by leptin. Biochem J 2006;393:7-20.