Alkaline phosphatase isoenzymes in children with respiratory diseases
Slavica Dodig
[*]
[1]
Jadranka Demirović
[2]
Žaneta Jelčić
[1]
Darko Richter
[1]
Ivana Čepelak
[3]
Miljenko Raos
[1]
Rajka Petrinović
[2]
Kornelija Kovač
[1]
Nina Peruško Matasić
[1]
Introduction
Alkaline phosphatase, orthophosphate-phosphohydrolase (ALP; EC 3.1.3.1) is a common term for a group of glycosylated enzymes (isoenzymes) with optimal activity in the alkaline range (pH 9.8–10.5). The isoenzymatic forms of different origin (liver, kidney, bone, placenta and intestine) have different physicochemical and immunologic characteristics that are employed in the methods of isoenzyme separation (1). The catalytic activity of ALP undergoes modification in childhood due to bone growth; it is moderately increased in the first three months of life, while in puberty it is two- to three-fold that in adults.
Transient hyperphosphatasemia (TH) in infants and children is a benign increase in the catalytic activity of serum ALP, which may persist for several weeks (2). TH was first described in 1954 (3). As these children show no clinical signs of a bone or liver metabolic disease (4), TH is detected incidentally, on routine laboratory work-up. TH generally occurs by the age of 5 years; however, recurrences in adults have been described (5). The diagnosis of TH is made when ALP values have significantly decreased or returned to reference range within 3–4 months (5-7).
As TH may occur in children with respiratory diseases (2), the aim of the study was to show the electrophoretic pattern of ALP isoenzymes in serum of children exhibiting an increased total ALP catalytic activity in the course of acute respiratory disease.
Subjects and Methods
Subjects
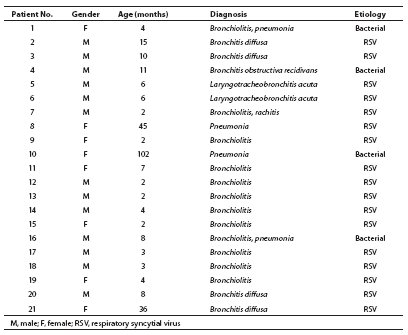
Results obtained in 21 children (17 or 81% of them infants), 13 male and eight female, aged 2 months to 8 years, are presented (Table 1). Because of the severity of acute respiratory disease (mostly bronchiolitis), study children were hospitalized at Srebrnjak Children’s Hospital in Zagreb between January 1 and February 28, 2007. In three children, radiological examination, leukocytosis and marked increase in the C-reactive protein (CRP) concentration pointed to bacterial etiology of their respiratory disease. The respiratory syncytial virus (RSV) infection was demonstrated in most children. Besides children with an increased total ALP catalytic activity, presentation is made of the children with bronchiolitis/pneumonia whose total ALP activity was within the reference range.
Table 1. General characteristics of study patients
Methods
Determination of alkaline phosphatase
Total ALP activity was determined by the recommended photometric continuous method with p-nitrophenylphosphate, AMP buffer, Mg acetate, Zn-sulfate, HEDTA (8).
Electrophoretic separation of alkaline phosphatase isoenzymes
Electrophoretic separation of ALP isoenzymes was performed on agarose gel (Hydragel 7/15 ISO-PAL gels) by use of the automated Hydrasys electrophoresis system, Hydrasys 2 densitometer (Sebia System, France), with serum lectin pretreatment (9).
Results
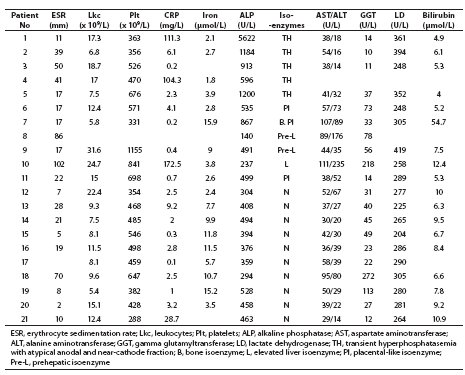
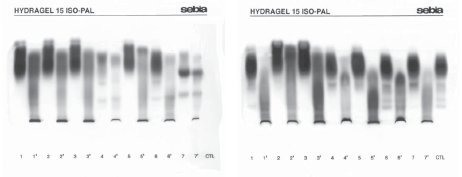
Increased total ALP catalytic activity (range, 528-5622 U/L) was found in eight (38.1%) children, and increased total activity of one or more other enzymes, i.e. aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma glutamyltransferase (GGT) and lactate dehydrogenase (LD), in 14 children. One month and a half upon completion of hospital treatment, total ALP activity was within the age-adjusted reference range in all study children. Electrophoretic separation of ALP isoenzymes showed normal age-specific isoenzyme pattern in ten (47.6%) children (Table 2, No. 12–21) (10). A characteristic TH picture with the appearance of fast anodal fraction (faster than hepatic fraction) and near-cathode fraction (faster than bone fraction) was recorded in five children (Table 2, No. 1–5). The placental-like isoenzyme was found in two children (No. 6 and 11), and bone fraction along with the placental-like isoenzyme fraction in one rachitic child (No. 7). Prehepatic isoenzymes were detected in two children (No. 8 and 9), whereas hepatic isoenzyme was found in one child (No. 10). Comparison of diluted patient samples (lanes 1–7) and lectin pretreated samples (lanes 1’–7’) is presented in Figure 1.
In the infant affected with bronchiolitis/pneumonia, total ALP catalytic activity (5622 U/L) decreased in 54 days to the reference range (349 U/L), while the isoenzyme pattern showed normal finding. In the rachitic child, the value of ALP decreased with vitamin D therapy.
Table 2 Analyte values in children during hospital stay (bold, analyte values exceeding reference range)
Figure 1 Electrophoretic separation of alkaline phosphatase (ALP) isoenzymes; lanes 1-7: diluted sera; lanes 1’-7’: the same serum samples pretreated with lectin.
A. Infants No. 1, 3 and 4 with transient hyperphosphatasemia (TH)
(1–3); No. 1 infant’s mother showing hepatic, intestinal and bone ALP (4); infant No. 2 with TH (5); child No. 21 showing hepatic, intestinal and bone ALP (6); pregnant woman showing hepatic, placental and bone ALP (7).
B. Control sample 1 (1); infant with TH (2, 3); infant showing bone isoenzyme and placental-like ALP isoenzyme (4, 7); infant No. 1 at control testing on day 54 showing hepatic, bone and intestinal ALP isoenzymes (6); control sample 2 (CTL).
Discussion
The study included children exhibiting increased total ALP catalytic activity in the course of acute respiratory disease, and varying isoenzyme patterns obtained by electrophoretic separation of ALP isoenzymes, i.e. 1) typical TH pattern; 2) placental-like isoenzyme; 3) prehepatic isoenzyme; 4) markedly increased hepatic isoenzyme; and 5) bone ALP isoenzyme expression in the rachitic child.
The criteria for the diagnosis of benign TH include age below 5 years; various symptoms such as respiratory infection, diarrhea and vomiting; no clinical or biochemical signs of bone or liver disease; increased total ALP values of 3- to 50-fold upper reference value for age; and return to the reference range within 4 months (2). Qualitatively and quantitatively, the isoenzymes display a specific pattern, i.e. increase in the activity of the fast anodal and near-cathode fraction (11). TH usually develops during the course of an infection (12), acute viral infections in particular (13,14), showing a male predominance (male to female ratio, 1.29:1) (15). TH may occur in mild respiratory or intestinal infections (16). Our infants with TH suffered from a serious acute respiratory disease of viral (RSV) or bacterial etiology. Suzuki et al. report elevated concentration of antibodies to Echo 22, Entero 71 and Coxsackie B4 enteroviruses in children with infectious diseases of upper airways accompanied by fever and diarrhea that developed TH (15). A case of rotavirus infection in a liver transplanted child has also been described (7). Parisi et al. report on TH in a patient with exanthematous fever following anti-measles vaccination (17). TH may occur in 2.8% of children with liver or kidney transplant (18). Placental isoenzyme cannot be detected in newborns (19). The occurrence of placental-like isoenzyme, the origin of which can only be speculated, has not yet been reported as a phenomenon accompanying respiratory disease in infants and children. It remains to determine whether it maybe derives from the thymus (20) or the lungs in the course of respiratory disease. The occurrence of prehepatic ALP isoenzyme and the increased activity of hepatic isoenzyme in our subjects may have been consequential to medicamentous therapy (the patients also showed increased catalytic activity of aminotransferases, GGT and LD), however, it should be additionally investigated.
Electrophoretic separation mostly identified isoenzymes of bone and hepatic origin in TH (21). The changed motility of hepatic and bone isoenzymes (anodal and near-cathode fraction) seems to be caused by the increased amount of sialic acid. The increase in the catalytic activity of ALP has been attributed to the possibly increased ALP synthesis due to the action of vitamin D metabolites, and in disease due to the possibly reduced hepatic clearance from the circulation caused by enhanced sialinization or hepatic effect of some drugs (21). However, the true origin of ALP isoenzymes cannot be assessed on the basis of electrophoretic separation. The finding of fragmented hepatic and bone isoenzymes may be consequential to a decreased clearance in the course of viral infection (22). Apart from transient hyperphosphatasemia, cases of permanent hyperphosphatasemia, which may be hereditary asymptomatic (23), non-hereditary (24), or in association with mental retardation (25), have also been reported.
To our knowledge, the occurrence of TH in Croatia was described in 1986 (26). How well is TH recognized? Or, is it simply ascribed to the physiological growth of bone mass? What is the prevalence of TH in children with acute infections? Is the ALP activity determined only in a minor proportion of children with infections?
The occurrence of increased ALP catalytic activity in an infant in the course of an acute respiratory infection points to the existence of benign TH. TH is subsequently verified, i.e. when the activity of ALP has significantly decreased and returned to the reference range. If the values of ALP normalize within 4 months, no additional testing is required (6,7).
Notes
Potential conflict of interest
None declared.
References
1. Štraus B. Medicinska biokemija. Medicinska naklada, Zagreb 1992.
2. Kraut JR, Metrick M, Maxwell NR, Kaplan MM. Isoenzyme studies in transient hyperphosphatasemia of infancy. Ten new cases and a review of the literature. Am J Dis Child 1985;139:736-40.
3. Bach U. Das Verhalten der alkalischen Serum-Phosphatase bei Fruhgeborenen, Rachitikern und Spasmophilen. Z Kinderheilkd 1954;74:593-609.
4. Kutilek S, Bayer M. Transient hyperphosphatasemia – where we stand? Turk J Pediatr 1999;41:151-60.
5. Onica D, Torssander J, Waldenlind L. Recurrent transient hyperphosphatasemia of infancy in an adult. Clin Chem 1992;38:1913-5.
6. Tolaymat N, deMelo MCN. Benign transient hyperphosphatasemia of infancy and childhood. South Med J 2000;93:1162-4.
7. Arikan C, Arslan MT, Kilic M, Aydogdu S. Transient hyperphosphatasemia after pediatric liver transplantation. Pediatr Int 2006;48:390-2.
8. Harmonizacija laboratorijskih nalaza u području opće medicinske biokemije. Hrvatska komora medicinskih biokemičara, Klinička bolnica Merkur, Zavod za kliničku kemiju, Zagreb 2004, p. 8.
9. Rosalki SB, Foo AY. Lectin affinity electrophoresis of alkaline phosphatase for the differentiation of bone and hepatobiliary disease. Electrophoresis 1989;10:604-11.
11. Behulova D, Bzduch V, Kasanicka A, Ticha L, Kucekova G. Electrophoresis of alkaline phosphatase isoenzymes – the key to rapid diagnosis in transient hyperphosphatemia. Cesk Pediatr 1993;48:193-5.
12. Oggero R, Mostert M, Spinello M, Iavarone A, Buffa J. Transient hyperphosphatasemia in infancy. Fifteen new cases. Acta Paediatr Scand 1988;77:257-9.
13. Eboriadou M, Skouli G, Panagopoulou P, Haidopoulou K, Makedou A, Varlamis G. Acute laryngotracheobronchitis and associated transient hyperphosphatasemia: a new case of transient hyperphosphatasemia in early childhood. J Pediatr Child Health 2006;42:149-50.
14. Griffiths J, Vernocchi A, Simoni E. Transient hyperphosphatasemia of infancy and childhood. A study of serum alkaline phosphatase by electrofocusing techniques. Arch Pathol Lab Med 1995;119:784-9.
15. Suzuki M, Okazaki T, Nagai T, Toro K, Setonyi P. Viral infection of infants and children with benign transient hyperphosphatasemia. FEMS Immunol Med Microbiol 2002;12:215-8.
16. Garty BZ, Nitzan M. Benign transient hyperphosphatasemia. Isr J Med Sci 1994;30:66-9.
17. Parisi G, Chiarelli A, Brandani M, D’Onofrio A. Transient alkaline hyperphosphatasemia in childhood. A report of 4 clinical cases and etiopathogenetic hypotheses. Minerva Pediatr 1991;43:337-41.
18. Ranchin B, Villard F, Andre JL, Canterino I, Said MH, Boisson RC, et al. Transient hyperphosphatasemia after organ transplantation in children. Pediatr Transplant 2002;6:308-12.
19. Crofton PM. Properties of alkaline phosphatase isoenzymes in plasma of preterm and term neonates. Clin Chem 1987;33:1778-82.
20. Sokolić B, Čepelak I. Izoenzimi i višestruki oblici alkalne fosfataze; značenje, mogućnosti određivanja. Biochemia Medica 1994;4:113-21.
21. Crofton PM. What is the cause of benign transient hyperphosphatasemia? A study of 35 cases. Clin Chem 1988;34:335-40.
22. Schoenau E,¸Herzog KH, Boehles HJ. “Fragmented” isoenzymes of alkaline phosphatase in the diagnosis of transient hyperphosphatasemia. Clin Chem 1986;32:2211-3.
23. Asami T, Gomi T, Uchijama M. Persistent non-familial asymptomatic hyperphosphatasemia: a report on three cases. Acta Pediatr 1995;84:346-8.
24. Wilson JW. Inherited elevation of alkaline phosphatase activity in the absence of disease. N Engl J Med 1979;301:983-4.
25. Kruse K, Hanefeld F, Kohlschutter A, Rosskamp R, Gross-Selbeck G. Hyperphosphatasia with mental retardation. J Pediatr 1988;112:436-9.
26. Jurčić Z, Rudar D, Hajnžić TF, Cvitanaović Lj, Babić M. Benigna tranzitorna hiperfosfatazemija. Knjiga sažetaka. V. Jugoslavensko-austrijski simpozij o bolestima jetre. Zadar, 1986:59.