References
1 Zachara NE, Hart GW. Cell signaling, the essential role of O-GlcNAc! Biochim Biophys Acta 2006;1761:599-617.
2 Torres CR, Hart GW. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J Biol Chem 1984;259:3308-17.
3 Wells L, Vosseller K, Hart GW. A role for N-acetylglucosamine as a nutrient sensor and mediator of insulin resistance. Cell Mol Life Sci 2003;60:222-8.
4 Slawson C, Zachara NE, Vosseller K, Cheung WD, Lane MD, Hart GW. Perturbations in O-linked beta-N-acetylglucosamine protein modification cause severe defects in mitotic progression and cytokinesis. J Biol Chem 2005;280:32944-56.
5 Lamarre-Vincent N, Hsieh-Wilson LC. Dynamic glycosylation of the transcription factor CREB: a potential role in gene regulation. J Am Chem Soc 2003;125:6612–3.
6 Jackson SP, Tjian R. O-glycosylation of eukaryotic transcription factors: implications for mechanisms of transcriptional regulation. Cell 1988; 55:125–33.
7 James LR, Tang D, Ingram A, Ly H, Thai K, Cai L, Scholey JW. Flux through the hexosamine pathway is a determinant of nuclear factor κB-dependent promoter activation. Diabetes 2002;51:1146–56.
8 Shaw P, Freeman J, Bovey R, Iggo R. Regulation of specific DNA binding by p53: evidence for a role for O-glycosylation and charged residues at the carboxy-terminus. Oncogene 1996;12:921–30.
9 Hiromura M, Choi CH, Sabourin NA, Jones H, Bachvarov D, Usheva A. YY1 is regulated by O-linked N-acetylglucosaminylation (O-GlcNAcylation). J Biol Chem 2003;278:14046–52.
10 Buse MG. Hexosamines, insulin resistance, and the complications of diabetes: current status. Am J Physiol Endocrinol Metab 2006;290:E1-E8.
11 Zachara NE, O’Donnell N, Cheung WD, Mercer JJ, Marth JD, Hart GW. Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells. J Biol Chem 2004;279:30133-42.
12 Fulop N, Marchase RB, Chatham JC. Role of protein O-linked N-acetylglucosamine in mediating cell function and survival in the cardiovascular system. Cardiovasc Res 2007;73:288-97.
13 Largo R, Alvarez-Soria MA, Diez-Ortego I, Calvo E, Sanchez-Pernaute O, Egido J, Herrero-Beaumont G. Glucosamine inhibits IL-1beta-induced NFkappaB activation in human osteoarthritic chondrocytes. Osteoarthritis Cartilage 2003;11:290-8.
14 Liu J, Pang Y, Chang T, Bounelis P, Chatham JC, Marchase RB. Increased hexosamine biosynthesis and protein O-GlcNAc levels associated with myocardial protection against calcium paradox and ischemia. J Mol Cell Cardiol 2006;40:303-12.
15 Snow DM, Hart GW. Nuclear and cytoplasmic glycosylation. Int Rev Cytol 1998;181:43-74.
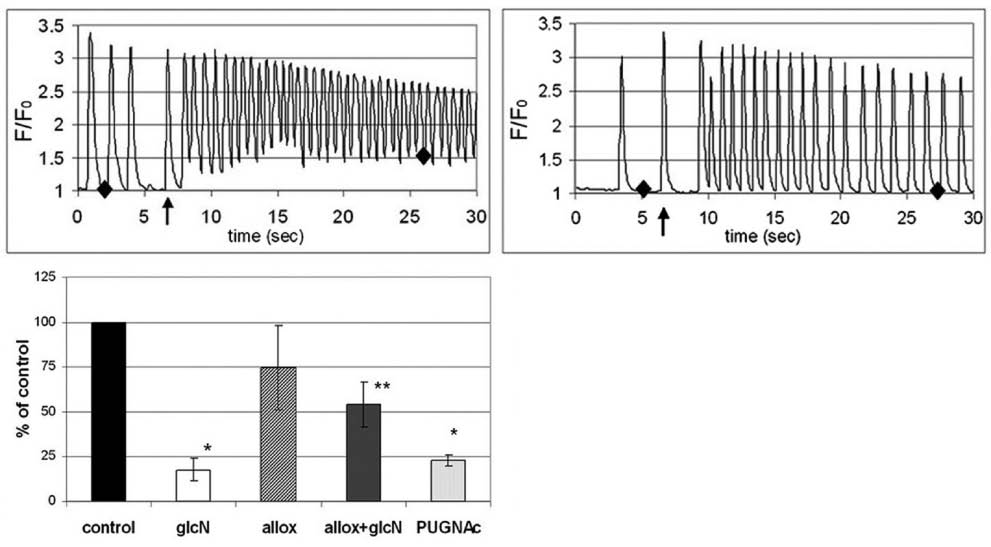
16 Nagy T, Champattanachai V, Marchase RB, Chatham JC. Glucosamine inhibits angiotensin II-induced cytoplasmic Ca2+ elevation in neonatal cardiomyocytes via protein-associated O-linked N-acetylglucosamine. Am J Physiol Cell Physiol 2006;290:C57-65.
17 Lefebvre T, Ferreira S, Dupont-Wallois L, Bussiere T, Dupire MJ, Delacourte A, et al. Evidence of a balance between phosphorylation and O-GlcNAc glycosylation of Tau proteins–a role in nuclear localization. Biochim Biophys Acta 2003;1619:167–76.
18 Kelly WG, Dahmus ME, Hart GW. RNA polymerase II is a glycoprotein. Modification of the COOH-terminal domain by O-GlcNAc. J Biol Chem 1993;268:10416–24.
19 Federici M, Menghini R, Mauriello A, Hribal ML, Ferrelli F, Lauro D, et al. Insulin-dependent activation of endothelial nitric oxide synthase is impaired by O-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Circulation 2002;106:466–72.
20 Reason AJ, Morris HR, Panico M, Marais R, Treisman RH, Haltiwanger RS, et al. Localization of O-GlcNAc modification on the serum response transcription factor. J Biol Chem 1992;267:16911–21.
21 Parker GJ, Lund KC, Taylor RP, McClain DA. Insulin resistance of glycogen synthase mediated by o-linked N-acetylglucosamine. J Biol Chem 2003;278:10022–7.
22 Shafi R, Lyer SP, Ellies LG, O’Donnell N, Marek KW, Chui D, et al. The O-GlcNAc transferase gene resides on the X chromosome and is essential for embryonic stem cell viability and mouse ontogeny. Proc Natl Acad Sci USA 2000;97:5735–9.
23 Kreppel LK, Blomberg MA, Hart GW. Dynamic glycosylation of nuclear and cytosolic proteins: cloning and characterization of a unique O-GlcNAc transferase with multiple tetratricopeptide repeats. J Biol Chem 1997;272:9308–15.
24 Wells L, Gao Y, Mahoney JA, Vosseller K, Chen C, Rosen A, Hart GW Dynamic O-glycosylation of nuclear and cytosolic proteins: further characterization of the nucleocytoplasmic beta-N-acetylglucosaminidase, O-GlcNAcase. J Biol Chem 2002;277:1755–61.
25 Comer FI, Vosseller K, Wells L, Accavitti MA, Hart GW. Characterization of a mouse monoclonal antibody specific for O-linked N-acetylglucosamine. Anal Biochem 2001;293:169-77.
26 Snow CM, Senior A, Gerace L. Monoclonal antibodies identify a group of nuclear pore complex glycoproteins. J Cell Biol 1987;104:1143-56.
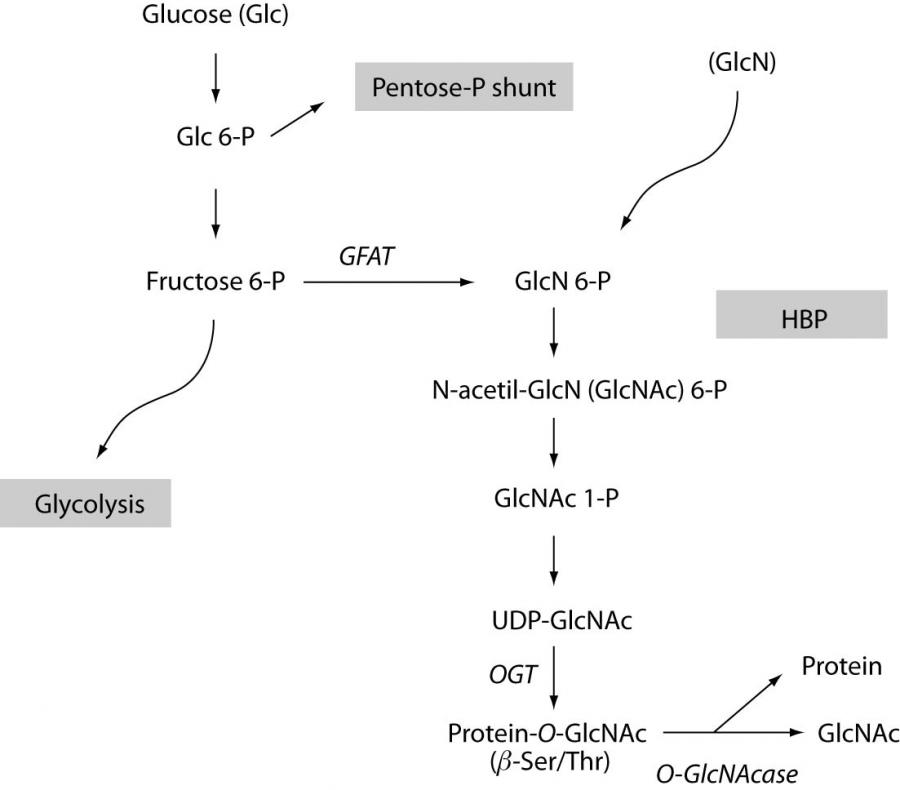
27 James LR, Fantus IG, Goldberg H, Ly H, Scholey JW. Overexpression of GFAT activates PAI-1 promoter in mesangial cells. Am J Physiol Renal Physiol 2000;279:F718-27.
28 McClain DA, Lubas WA, Cooksey RC, Hazel M, Parker GJ, Love DC, Hanover JA. Altered glycan-dependent signaling induces insulin resistance and hyperleptinemia. Proc Natl Acad Sci USA 2002;99:10695–9.
29 Clark RJ, McDonough PM, Swanson E, Trost SU, Suzuki M, Fukuda M, Dillmann WH. Diabetes and the accompanying hyperglycemia impairs cardiomyocyte calcium cycling through increased nuclear O-GlcNAcylation. J Biol Chem 2003;278:44230-7.
30 Hresko RC, Heimberg H, Chi MM, Mueckler M. Glucosamine-induced insulin resistance in 3T3-L1 adipocytes is caused by depletion of intracellular ATP. J Biol Chem 1998;273:20658-68.
31 Liu J, Marchase RB, Chatham JC. Glutamine-induced protection of isolated rat heart from ischemia/reperfusion injury is mediated via the hexosamine biosynthesis pathway and increased protein O-GlcNAc levels. J Mol Cell Cardiol 2007;42:177-85.
32 Konrad RJ, Zhang F, Hale JE, Knierman MD, Becker GW, Kudlow JE. Alloxan is an inhibitor of the enzyme O-linked N-acetylglucosamine transferase. Biochem Biophys Res Commun. 2002;293:207-12.
33 Haltiwanger RS, Grove K, Philipsberg GA. Modulation of O-linked N-acetylglucosamine levels on nuclear and cytoplasmic proteins in vivo using the peptide O-GlcNAc-beta-N-acetylglucosaminidase inhibitor O-(2-acetamido-2-deoxy-D-glucopyranosylidene)amino-N-phenylcarbamate. J Biol Chem 1998;273:3611-7.
34 Konrad RJ, Mikolaenko I, Tolar JF, Liu K, Kudlow JE. The potential mechanism of the diabetogenic action of streptozotocin: inhibition of pancreatic β-cell O-GlcNAc-selective N-acetyl-β-D-glucosaminidase. Biochem J 2001;356:31–41.
35 Griffith LS, Schmitz B. O-linked N-acetylglucosamine levels in cerebellar neurons respond reciprocally to pertubations of phosphorylation. Eur J Biochem 1999;262:824–31.
36 Vosseller K, Wells L, Lane MD, Hart GW. Elevated nucleocytoplasmic glycosylation by O-GlcNAc results in insulin resistance associated with defects in Akt activation in 3T3-L1 adipocytes. Proc Natl Acad Sci USA 2002;99:5313–8.
37 Wells L, Kreppel LK, Comer FI, Wadzinski BE, Hart GW. OglcNAc transferase is in a functional complex with protein phosphatase 1 catalytic subunits. J Biol Chem 2004;279:38466–70.
38 Robertson LA, Moya KL, Breen KC. The potential role of tau protein Oglycosylation in Alzheimer’s disease. J Alzheimers Dis 2004;6:489–95.
39 Fulop N, Zhang Z, Marchase RB, Chatham JC. Glucosamine cardioprotection in perfused rat heart associated with increased O-Linked N-acetylglucosamine protein modification and altered p38 activation. Am J Physiol Heart Circ Physiol 2007;292:H2227-36.
40 Cheng X, Hart GW. Alternative O-glycosylation/O-phosphorylation of serine-16 in murine estrogen receptor beta: posttranslational regulation of turnover and transactivation activity. J Biol Chem 2001;276:10570–5.
41 Han I, Kudlow JE. Reduced O-glycosylation of Sp1 is associated with increased proteasome susceptibility. Mol Cell Biol 1997;17:2550–8.
42 Zhang F, Su K, Yang X, Bowe DB, Paterson AJ, Kudlow JE. OglcNAc modification is an endogenous inhibitor of the proteasome. Cell 2003;115:715–25.
43 Kamemura K, Hart GW. Dynamic interplay between O-glycosylation and O-phosphorylation of nucleocytoplasmic proteins: a new paradigm for metabolic control of signal transduction and transcription. Prog Nucleic Acid Res Mol Biol 2003;73:107-36.
44 Hawkins M, Barzilai N, Liu R, Hu M, Chen W, Rossetti L. Role of the glucosamine pathway in fat-induced insulin resistance. J Clin Invest 1997;99:2173–82.
45 Marshall S, Bacote V, Traxinger RR. Complete inhibition of glucose-induced desensitization of the glucose transport system by inhibitors of mRNA synthesis. Evidence for rapid turnover of glutamine:fructose-6-phosphate amidotransferase. J Biol Chem 1991;266: 10155–61.
46 Rossetti L, Hawkins M, Chen W, Gindi J, Barzilai N. In vivo glucosamine infusion induces insulin resistance in normoglycemic but not in hyperglycemic conscious rats. J Clin Invest 1995;96:132–40.
47 Robinson KA, Sens DA, Buse MG. Pre-exposure to glucosamine induces insulin resistance of glucose transport and glycogen synthesis in isolated rat skeletal muscles. Study of mechanisms in muscle and in rat-1 fibroblasts overexpressing the human insulin receptor. Diabetes 1993;42:1333–46.
48 Ross SA, Chen X, Hope HR, Sun S, McMahon EG, Broschat K et al. Development and comparison of two 3T3-L1 adipocyte models of insulin resistance: increased glucose flux vs glucosamine treatment. Biochem Biophys Res Commun 2000;273: 1033–41.
49 Parker G, Taylor R, Jones D, McClain D. Hyperglycemia and inhibition of glycogen synthase in streptozotocin-treated mice: role of O-linked N-acetylglucosamine. J Biol Chem 2004;279:20636–42.
50 Oriente F, Formisano P, Miele C, Fiory F, Maitan MA, Vigliotta G, et al. Insulin receptor substrate-2 phosphorylation is necessary for protein kinase C zeta activation by insulin in L6hIR cells. J Biol Chem 2001;276:37109-19.
51 Kamemura K, Hayes BK, Comer FI, Hart GW. Dynamic interplay between O-glycosylation and O-phosphorylation of nucleocytoplasmic proteins: alternative glycosylation/phosphorylation of THR-58, a known mutational hot spot of c-Myc in lymphomas, is regulated by mitogens. J Biol Chem 2002;277:19229–35.
52 Walgren JL, Vincent TS, Schey KL, Buse MG. High glucose and insulin promote O-GlcNAc modification of proteins, including α-tubulin. Am J Physiol Endocrinol Metab 2003;284:424–34.
53 King IA, Hounsell EF. Cytokeratin 13 contains O-glycosidically linked N-acetylglucosamine residues. J Biol Chem 1989;264:14022–8.
54 Chou CF, Smith AJ, Omary MB. Characterization and dynamics of O-linked glycosylation of human cytokeratin 8 and 18. J Biol Chem 1992;267:3901-6.
55 Hiromura M, Choi CH, Sabourin NA, Jones H, Bachvarov D, Usheva A. YY1 is regulated by O-linked N-acetylglucosaminylation (O-GlcNAcylation). J Biol Chem 2003;278:14046–52.
56 Boehmelt G, Wakeham A, Elia A, Sasaki T, Plyte S, Potter J, et al. Decreased UDP-GlcNAc levels abrogate proliferation control in EMeg32-deficient cells. EMBO J 2000;19:5092-104.
57 Kelly WG, Hart GW. Glycosylation of chromosomal proteins: localization of O-linked N-acetylglucosamine in Drosophila chromatin. Cell 1989;57:243–51.
58 Hanover JA, Cohen CK, Willingham MC, Park MK. O-linked N-acetylglucosamine is attached to proteins of the nuclear pore. Evidence for cytoplasmic and nucleoplasmic glycoproteins. J Biol Chem 1987;262:9887–94.
59 Miller MW, Hanover JA. Functional nuclear pores reconstituted with beta 1-4 galactose-modified O-linked N-acetylglucosamine glycoproteins. J Biol Chem 1994;269:9289–97.
60 Whelan SA, Hart GW. Proteomic approaches to analyze the dynamic relationships between nucleocytoplasmic protein glycosylation and phosphorylation. Circ Res 2003;93:1047-58.
61 Roos MD, Su K, Baker JR, Kudlow JE. O-Glycosylation of an Sp1-derived peptide blocks known Sp1 protein interactions. Mol Cell Biol 1997;17:6472–80.
62 Goldberg HJ, Whiteside CI, Hart GW, Fantus IG. Posttranslational, reversible O-glycosylation is stimulated by high glucose and mediates plasminogen activator inhibitor-1 gene expression and Sp1 transcriptional activity in glomerular mesangial cells. Endocrinology 2006;147:222-31 Erratum in: Endocrinology 2006;147:5490.
63 Freund C, Schmidt-Ullrich R, Baurand A, Dunger S, Schneider W, Loser P, et al. Requirement of nuclear factor-kappaB in angiotensin II- and isoproterenol-induced cardiac hypertrophy in vivo. Circulation 2005;111:2319-25.
64 Umezawa K. Inhibition of tumor growth by NF-kappaB inhibitors. Cancer Sci 2006;97:990-5.
65 Kim YH, Song M, Oh YS, Heo K, Choi JW, Park JM, et al. Inhibition of phospholipase C-beta1-mediated signaling by O-GlcNAc modification. J Cell Physiol 2006;207:689-96.
66 Schipke JD, Friebe R, Gams E. Forty years of glucose-insulin-potassium (GIK) in cardiac surgery: a review of randomized, controlled trials. Eur J Cardiothorac Surg 2006;29:479-85.
67 Schaffer SW, Croft CB, Solodushko V. Cardioprotective effect of chronic hyperglycemia: effect on hypoxia-induced apoptosis and necrosis. Am J Physiol Heart Circ Physiol 2000;278:H1948-54.
68 Hunton DL, Lucchesi PA, Pang Y, Cheng X, Dell’Italia LJ, Marchase RB. Capacitative calcium entry contributes to nuclear factor of activated T-cells nuclear translocation and hypertrophy in cardiomyocytes. J Biol Chem 2002;277:14266-73.
69 Bush EW, Hood DB, Papst PJ, Chapo JA, Minobe W, Bristow MR, et al. Canonical transient receptor potential channels promote cardiomyocyte hypertrophy through activation of calcineurin signaling. J Biol Chem 2006;281:33487-96.
70 Buse MG, Robinson KA, Marshall BA, Hresko RC, Mueckler MM. Enhanced O-GlcNAc protein modification is associated with insulin resistance in GLUT1-overexpressing muscles. Am J Physiol Endocrinol Metab 2002;283:241–50.
71 Andreozzi F, D’Alessandris C, Federici M, Laratta E, Del Guerra S, Del Prato S, et al. Activation of the hexosamine pathway leads to phosphorylation of insulin receptor substrate-1 on Ser307 and Ser612 and impairs the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin insulin biosynthetic pathway in RIN pancreatic beta-cells. Endocrinology 2004;145:2845–57.
72 Park SY, Ryu J, Lee W. O-GlcNAc modification on IRS-1 and Akt2 by PUGNAc inhibits their phosphorylation and induces insulin resistance in rat primary adipocytes. Exp Mol Med 2005;37:220-9.
73 Chen G, Liu P, Thurmond DC, Elmendorf JS. Glucosamine-induced insulin resistance is coupled to O-linked glycosylation of Munc18c. FEBS Lett 2003;534:54–60.
74 Robinson KA, Ball LE, Buse MG. Reduction of O-GlcNAc protein modification does not prevent insulin resistance in 3T3-L1 adipocytes. Am J Physiol Endocrinol Metab 2007;292:E884-90.
75 Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001;414:813-20.
76 Daniels MC, Kansal P, Smith TM, Paterson AJ, Kudlow JE, McClain DA. Glucose regulation of transforming growth factor-alpha expression is mediated by products of the hexosamine biosynthesis pathway. Mol Endocrinol 1993;7:1041–8.
77 Kolm-Litty V, Sauer U, Nerlich A, Lehmann R, Schleicher ED. High glucose-induced transforming growth factor beta1 production is mediated by the hexosamine pathway in procine glomerular mesangial cells. J Clin Invest 1998;101:160–9.
78 Weigert C, Brodbeck K, Sawadogo M, Haring HU, Schleicher ED. Upstream stimulatory factor (USF) proteins induce human TGF-beta1 gene activation via the glucose-response element-1013/-1002 in mesangial cells: up-regulation of USF activity by the hexosamine biosynthetic pathway. J Biol Chem 2004;279:15908–15.
79 Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Invest 2001;108:1341–8.
80 Matthews JA, Acevedo-Duncan M, Potter RL. Selective decrease of membrane-associated PKC-alpha and PKC-epsilon in response to elevated intracellular O-GlcNAc levels in transformed human glial cells. Biochim Biophys Acta 2005;1743:305-15.
81 Yang S, Zou LY, Bounelis P, Chaudry I, Chatham JC, Marchase RB. Glucosamine administration during resuscitation improves organ function after trauma hemorrhage. Shock 2006;25:600-7.
82 Champattanachai V, Marchase RB, Chatham JC. Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein-associated O-GlcNAc. Am J Physiol Cell Physiol 2007;292:C178-87.
83 Roquemore EP, Chevrier MR, Cotter RJ, Hart GW. Dynamic O-GlcNAcylation of the small heat shock protein αB-crystallin. Biochemistry 1996;35:3578–86.
84 Wright DC, Geiger PC, Holloszy JO, Han DH. Contraction- and hypoxia-stimulated glucose transport is mediated by a Ca2+-dependent mechanism in slow-twitch rat soleus muscle. Am J Physiol Endocrinol Metab 2005;288:E1062-6.
85 Bolli R. Preconditioning: a paradigm shift in the biology of myocardial ischemia. Am J Physiol Heart Circ Physiol 2007;292:H19-27.
86 Hausenloy DJ, Yellon DM. Survival kinases in ischemic preconditioning and postconditioning. Cardiovasc Res 2006;70:240-53.
87 Marshall PD, Poddar S, Tweed EM, Brandes L. Clinical inquiries: Do glucosamine and chondroitin worsen blood sugar control in diabetes? J Fam Pract 2006;55:1091-3.
88 Chen JT, Chen CH, Horng CT, Chien MW, Lu DW, Liang JB, et al. Glucosamine sulfate inhibits proinflammatory cytokine-induced ICAM-1 production in human conjunctival cells in vitro. J Ocul Pharmacol Ther 2006;22:402-16.
89 Forchhammer L, Thorn M, Met O, Gad M, Weidner MS, Claesson MH. Immunobiological effects of glucosamine in vitro. Scand J Immunol 2003;58:404-11.
90 Ma L, Rudert WA, Harnaha J, Wright M, Machen J, Lakomy R, et al. Immunosuppressive effects of glucosamine. J Biol Chem 2002;277:39343-9.
91 Chou TY, Hart GW. O-linked N-acetylglucosamine and cancer: messages from the glycosylation of c-Myc. Adv Exp Med Biol 2001;491:413-8.
92 Yang WH, Kim JE, Nam HW, Ju JW, Kim HS, Kim YS, Cho JW. Modification of p53 with O-linked N-acetylglucosamine regulates p53 activity and stability. Nat Cell Biol 2006;8:1074-83.
93 Kanai M, Wei D, Li Q, Jia Z, Ajani J, Le X, et al. Loss of Kruppel-like factor 4 expression contributes to Sp1 overexpression and human gastric cancer development and progression. Clin Cancer Res 2006;12:6395-402.
94 Lefebvre T, Pinte S, Guerardel C, Deltour S, Martin-Soudant N, Slomianny MC, et al. The tumor suppressor HIC1 (hypermethylated in cancer 1) is O-GlcNAc glycosylated. Eur J Biochem 2004;271:3843-54.
95 Yang X, Zhang F, Kudlow JE. Recruitment of O-GlcNAc transferase to promoters by corepressor mSin3A: coupling protein O-GlcNAcylation to transcriptional repression. Cell 2002;110:69-80.