Introduction
Acid-base balance and electrolyte impairments are physiologically and clinically related, and are frequently encountered in clinical practice. A rapid and accurate diagnosis, and appropriate management of these impairments are of utmost importance (1,2).
Technological advances, sensor technology in particular, have resulted in the development of analyzers that provide fast and simple determination of these analytes both in laboratory setting and as point of care testing (POCT) (3,4). Electrochemical sensors have found application in clinical chemistry because of their simple use, minimal maintenance required, and ability to measure clinically relevant analytes in whole blood samples across a wide concentration range. The development of miniature sensors and their integration in small sophisticated analyzers have enabled evolution of POCT analyzers, thus bringing test analyses close to the patient in order to obtain test results within the shortest possible time, ensuring early therapy initiation and improved patient care in general (3). POCT has become available in hospitals as an alternative to central laboratory. This refers to intensive care units (ICU) in particular, where efficacious patient management requires regular monitoring of the acid-base balance parameters and electrolytes, as well as to other hospital settings requiring close monitoring of acute and chronic states (3,4). The spectrum of POCT analyzers has been continuously expanding towards ever faster and thorough patient care and examination, thus improving both clinical and economic aspects of treatment (4).
GEM Premier 3000
The GEM Premier 3000 analyzer evaluated in the present study is a portable analyzer for rapid POCT analysis of whole blood samples, manufactured by Instrumentation Laboratory Company, Lexington, MA, USA. This POCT analyzer provides simultaneous determination of the following analytes: pH, pCO2, pO2, Na+, K+, Ca++, glucose, lactate, and hematocrit. Determination of analyte concentration is based on potentiometry (pH, pCO2, Na+, K+ and Ca++), amperometry (pO2, glucose and lactate), and conductometry (hematocrit). The analyzer is intended for use by health professionals in any type of hospital setting and is designed in concordance with European standards.
The analyzer consists of two components, i.e. the instrument and the replaceable cartridge as a primary component. The replaceable cartridge is a closed system that contains all components needed for sample analysis, i.e. the needle for sampling, sensor card, calibration/control solutions, lines, valves, pumps, and waste container. Cartridge insertion in the analyzer is followed by automated sensor calibration. External validation of the calibration and cartridge accuracy is performed by original samples upon automated calibration and before patient sample analysis. Subsequent calibrations are performed automatically at preset time intervals in order to ensure constant accuracy of the analyzer performance. Three types of calibration are automatically performed, i.e. single-point calibration, double-point calibration, and oxygen calibration. Single-point calibration is performed automatically following each individual sample.
The manufacturer points to the exclusive program of active automated performance quality control termed Intelligent Quality Management (iQMTM). The iQMTM program has replaced conventional internal performance quality control, which has now become an integral part of the operating system, performing control of all measured parameters after each individual sample without user's intervention. The iQMTM program is started automatically upon properly performed validation of automated cartridge calibration. The program continually controls the entire process of sample analysis (including sensors, solutions and electronics), enables instantaneous error detection, and performs corrective actions for error elimination.
Another exclusive program is Failure Pattern Recognition Checks (FPRC), which helps in the identification of microthrombi, some errors in sensor performance, and interferences. During sample analysis, the GEM Premier 3000 analyzer performs automated control of microthrombi and interferences.
There are various types of cartridges that differ in analyte configuration (pH, blood gases/hematocrit/electrolytes/metabolites), number of tests (75–600, irrespective of the number of calibrations and controls), and duration (2 or 3 weeks). The sample to analyze can be arterial, capillary or venous blood, and Li–/Na-heparin in final concentration of 25 IU/mL is the only anticoagulant acceptable. Other anticoagulants such as EDTA, citrate, oxalate and NaF may cause damage to the sensors and are not recommended for use. The blood volume for analysis is 135–150 μL, and the time of sample analysis is 85 seconds.
The following analyzers fitted at central laboratory were used in the assessment of clinical characteristics of the study analyzer: Ciba Corning 865, Vitros 250, and Olympus AU 640.
Ciba Corning 865 analyzer
This analyzer is intended for determination of acid-base balance parameters, electrolytes, metabolites, hemoglobin and hemoglobin derivatives. In the present study, Ciba Corning 865 analyzer served as a reference analyzer for comparison determination of pH, pCO2, pO2, K+, Na+ and Ca++ in patient samples. The measurement technology for study analytes is based on electrochemical phenomenon (ISE technology, amperometry and potentiometry). Hematocrit cannot be determined on this analyzer, therefore this analyte was not submitted to analytical evaluation. The analyzer is manufactured by Ciba Corning Diagnostics Corporation, MA, USA.
Vitros 250 analyzer
It is a discrete biochemistry analyzer operating on the dry chemistry principle. In the present study, Vitros 250 analyzer served as a reference analyzer for comparison determination of lactate concentration. Plasma lactate concentration is determined by spectrophotometry. The analyzer is manufactured by Ortho Clinical Diagnostics, NY, USA.
Olympus AU 640 analyzer
It is an open, discrete, multi-channel biochemistry analyzer manufactured by Olympus Optical Co., Ltd., Tokyo, Japan, which served as a reference analyzer for comparison determination of glucose concentration in patient samples. Glucose concentration is determined by the method of enzymatic UV assay (hexokinase method).
All three analyzers are regularly submitted to performance quality control (internal and external) and maintenance procedures in line with the manufacturers’ instructions.
Aim
The aim of the study was to evaluate analytical properties and technical characteristics of the GEM Premier 3000 analyzer before its fitting and performance at University Department of Surgery ICU, and to determine the level of compatibility between the results obtained on this analyzer and those produced by the analyzers used at central laboratory, with due consideration of the specific clinical setting and analyzer utilization by non-laboratory personnel.
Analytical evaluation was performed by determination of within-run imprecision, between-run imprecision, inaccuracy, and concurrent determination of analyte concentrations in patient samples on the study analyzer and reference analyzers used for the respective analyte at central laboratory, included in the program of external quality control. All tests were performed according to recommendations issued by the National Committee for Clinical Laboratory Standards (NCCLS), Document EP09-A2 (4). As POCT has been gaining importance in clinical laboratory diagnosis and undergoes fastest technological advances, the study analyzer is expected to prove superior to classic laboratory analyzers according to some of its characteristics.
Materials and methods
Analyzers
GEM Premier 3000
Cartridge insertion is followed by automated sensor calibration. Upon automated calibration and before sample analysis, external validation of the calibration and cartridge is performed by use of original samples, Calibration Validation Products (CVP, Instrumentation Laboratory Company, Lexington, MA, USA). Subsequent calibrations are performed automatically at certain time intervals. Upon proper validation of automated calibration of the cartridge inserted, performance quality control is automatically taken over by iQMTM program using control solutions contained in the cartridge.
Ciba Corning 865 analyzer
Original calibration solutions (Buffer 6.838 and Buffer 7.3/COox Zero) are used for calibration, and original control samples Rapid QC Complete Level 1, 2 and 3 employed for performance quality control. The calibration and control solutions are manufactured by Bayer HealthCare LLC, MA, USA.
Vitros 250 analyzer
A multilayer film (Vitros Lac Slide) was used to determine lactate concentration in plasma; original calibrator (Calibrator kit 1) was used on calibration; and control samples Performance Verifier I and II were used on performance quality control. The films, calibration and control solutions are manufactured by Ortho Clinical Diagnostics, NY, USA.
Olympus AU 640 analyzer
Glucose reagent was used on glucose determination, System Calibrator was used on calibration, and control samples Olympus Control Level 1 and 2 were used on internal quality control. The reagent, calibrator and control samples are manufactured by Olympus Life and Material Science Europe GmbH, Hamburg, Germany.
Evaluation of analytical characteristics of the GEM Premier 3000 analyzer
Within-run imprecision study
Within-run imprecision was assessed by multiple (20 times) concentration determination of the pH, pCO2, pO2, K+, Na+ and Ca++, glucose and lactate analytes in samples of the Critical Care QC Contril 9 Multipak control solutions containing three levels (Level 1, 2 and 3; Instrumentation Laboratory Company, Lexington, MA, USA).
Between-run imprecision study
Between-run imprecision was assessed by determination of the same analyte concentrations for 20 days in samples of the control solutions Rapid QC Complete Level 1, 2 and 3; Bayer HealthCare LLC, MA, USA.
Inaccuracy study
Testing for inaccuracy was performed by determination of the pH, pCO2, pO2, K+, Na+ and Ca++, glucose and lactate analytes in samples of the Critical Care QC Contril 9 Multipak control solutions containing three levels (Level 1, 2 and 3; Instrumentation Laboratory Company, Lexington, MA, USA).
Concurrent determination of acid-base balance parameters and electrolyte concentration
A total of 160 whole blood (arterial, venous and mixed blood) samples were analyzed on the GEM Premier 3000 analyzer and Ciba Corning 865 (analytes: pH, pCO2, pO2, K+, Na+ and Ca++) as a reference analyzer. Blood samples collected from patients where determination of blood gases and pH was requested were used in the study, thus requiring no additional blood sampling. Blood samples were collected in heparinized syringes and analyzed simultaneously on both analyzers immediately upon receipt, in order to avoid changes in gas concentrations that may occur on sample storage.
Concurrent determination of glucose and lactate concentration
Fifty patient plasma samples (Li-heparin) were analyzed on the Olympus AU 640 (for glucose) and Vitros 250 analyzer (for lactate) as reference analyzers and on GEM Premier 3000. Blood samples collected from patients where determination of plasma glucose and lactate were requested were used in the study, thus requiring no additional blood sampling.
Statistical analysis
Statistical analysis of the results was performed by use of MedCalc Version 9.0.1.1 (Frank Schoonjans, Belgium) software. The mean (x), standard deviation (SD) and coefficient of variation (CV%) were calculated for assessment of imprecision. Percentage of the mean measured value deviation from the mean declared value (R%) was calculated for assessment of inaccuracy (5). The level of result correlation was expressed by Pearson’s correlation coefficient (r) with calculation (95%) of confidence intervals (CI) for each of the study analytes. Passing Bablok regression analysis of results was used to calculate the equation of direction and 95% CI for the slope (b) and y-axis intercept (a) for each of the study analytes. The criteria for the results to be considered acceptable were as follows:
1 for imprecision assessment, CV lower than 5.00%;
2 for inaccuracy assessment, deviation of the mean measured value from the mean declared value lower than 5.00%; and
3 for assessment of the level of result correlation, correlation coefficient greater than 0.9500.
Results
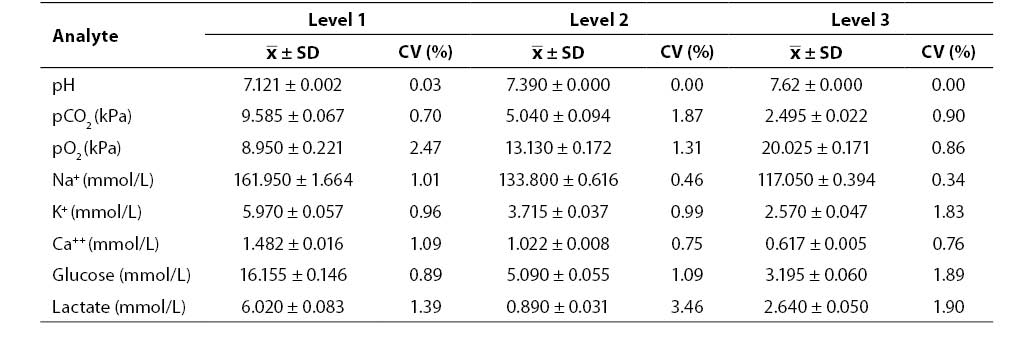
Results of the within-run imprecision study (mean, SD, CV; N = 20; Critical Care QC Contril 9 Multipak control sample Level 1, 2 and 3) are shown in Table 1. Results of the within-run imprecision study were satisfactory for all the analytes investigated (CV ≤ 3.46%).
Table 1. Results of within-run imprecision testing
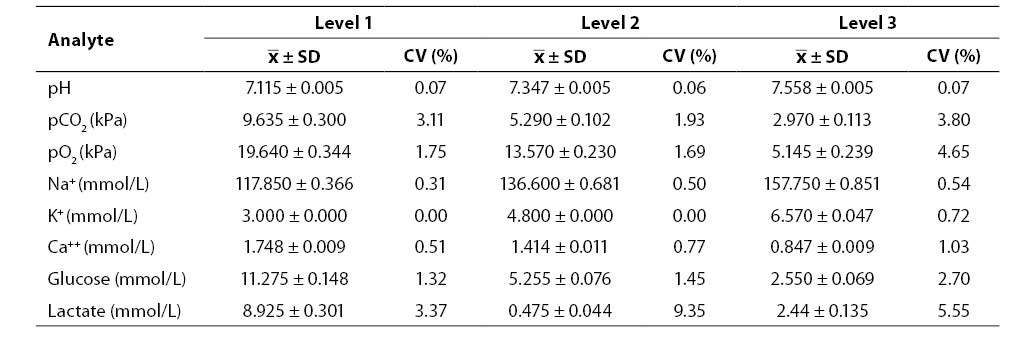
Results of the between-run imprecision study (mean, SD, CV; N = 20; Rapid QC Complete control sample Level 1, 2 and 3) are shown in Table 2. Between-run imprecision was satisfactory for all the analytes investigated (CV ≤4.65%) with the exception of lactate (Level 2, CV = 9.35%; Level 3, CV = 5.55%).
Table 2. Results of between-run imprecision testing
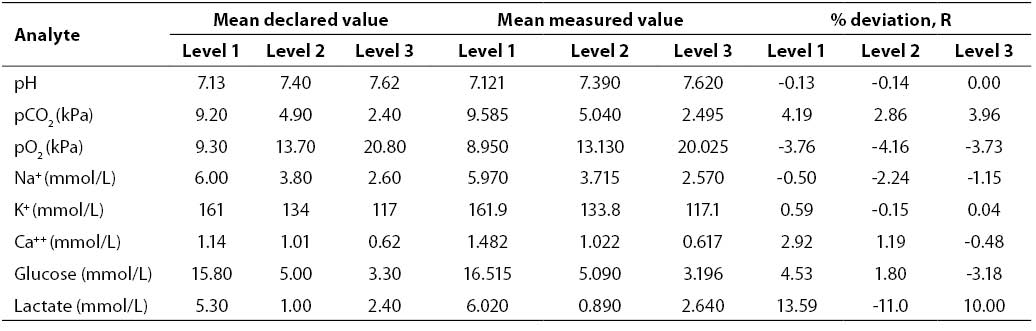
Results of the inaccuracy study (mean declared value, mean measured value, R; N = 20; Critical Care QC Contril 9 Multipak control sample Level 1, 2 and 3) are shown in Table 3. Results of the inaccuracy study were satisfactory for all the analytes investigated (R ≤4.53%) with the exception of lactate (Level 1, R = 13.59%; Level 2, R = -11.00%; Level 3, R = 10.00%).
Table 3. Results of inaccuracy testing
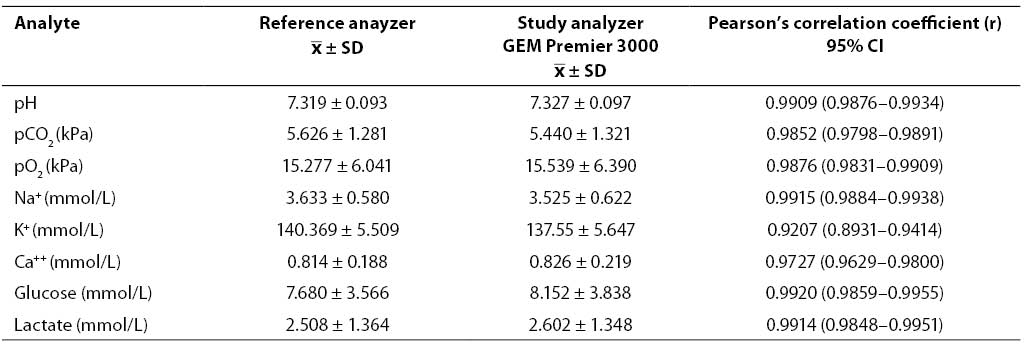
Results of concurrent determination of study analyte concentrations in patient samples on the GEM Premier 3000 analyzer and reference analyzers (mean, SD) as well as the calculated Pearson's coefficients of correlation (r) are shown in Table 4. The coefficients of correlation obtained showed high correlation for all analytes except sodium.
Table 4. Results of concurrent determination of analyte concentrations in patient samples and coefficients of correlation
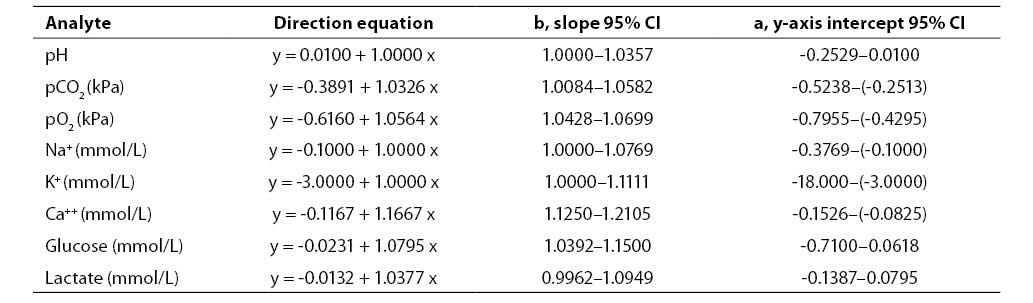
Results of concurrent determination of analyte concentrations in patient samples processed by Passing Bablok regression analysis are presented in Table 5. The results showed high compatibility between the study analyzer and reference analyzers.
Table 5. Results of Passing-Bablok regression analysis
Discussion
Assessment of the study analyzer GEM Premier 3000 revealed the within-run imprecision, between-run imprecision and inaccuracy to meet the preset criteria of acceptability for the majority of analytes (CV < 5.00%; R < 5.00%) at all the three analyte concentration levels evaluated. Correlation of the results obtained on the study analyzer and reference analyzers also satisfied the criteria of acceptability for most of the analytes evaluated (r>0.95).
The preset criteria of acceptability were not met in the study of between-run imprecision for lactate (Level 2 and 3), of inaccuracy for lactate (all three levels) and of coefficient of correlation for sodium.
A limitation of the present assessment was the fact that at the time of assessment (2006), the Ciba Corning 865 (manufactured in 1996) and Vitros 250 (manufactured in 1998) analyzers were used as reference analyzers. This may have been the reason for some of the results being found unacceptable (sodium and lactate).
Analytical characteristics of the GEM Premier 3000 analyzer have also been investigated by other authors. Beneteau-Burnat et al. (6) compared GEM Premier 3000 and Radiometer®ABL 725 analyzers. They assessed imprecision using aqueous control samples (ContrIL 9 Multipak manufactured by Instrumentation Laboratory Company, Lexington, MA, USA). Testing for within-run imprecision and between-run imprecision produced satisfactory coefficients of variation for all study analytes (borderline results were obtained for glucose and lactate at particular levels). Coefficients of variation obtained by comparison determination of patient samples on both instruments (N = 110) ranged from 0.91 to 0.99 (sodium r = 0.94 and ionized calcium r = 0.91). Regression analysis showed no result differences between the two analyzers either (6).
Steinfelder-Visscher et al. compared analytical characteristics of the GEM Premier 3000 and Ciba Corning 865 analyzers. Comparison of the results obtained in 127 patient whole blood samples yielded satisfactory results for all study analytes except for potassium (r = 0.79) (7).
The GEM Premier 3000 analyzer is simple to use. As the analyzer testing was performed at a clinical laboratory, the study required minimal training of the personnel. During the study, there was no need of service intervention, as there are no consumable parts, and the device requires minimal maintenance procedures. The exchangeable cartridge is a closed system that is periodically replaced (depending on the number of analyses/cartridge duration). The waste container is an integral part of the cartridge, thus contributing to the biological safety of the operator by minimizing the operator’s contact with blood samples. Different cartridge configurations (different test choice, number of tests and cartridge duration) add to the user’s flexibility and cost effectiveness.
The active automated performance quality control (calibration and control after each individual sample requiring no user’s manipulation) provides considerably faster error detection (within 30 minutes at the latest), which is a significant advantage over classic devices where the performance quality control is carried out every eight hours. Error detection is followed by independent error elimination and documentation of the corrective action or disconnection from performance of the sensor that has not undergone control before error elimination. This continuous automated control ensures safe performance and reliable results at any time.
The quality of POCT depends on preanalytical, analytica and postanalytical factors. The problems encountered on device operation are mostly caused by preanalytical errors. Close collaboration between laboratory and clinical personnel, and proper education of the staff performing POCT on the potential preanalytical errors are necessary for reliable and quality results.
The GEM Premier 3000 analyzer requires no specific setting conditions (work temperature 15–35 ºC, relative humidity 5%–90%); barometer pressure variation has no impact on the device performance.
The computer enables connection of the analyzer to the existing laboratory information system and transfer of results. The analyzer can be connected to the oximetry module and coagulation module. By use of UPS device, the analyzer can operate for up to one hour without electricity supply.
Conclusion
The high correlation of the results (except for sodium), and precision and accuracy (except for lactate) obtained on the GEM Premier 3000 analyzer indicated full compatibility with the analyzers used at central laboratory. Simple operation that does not require specially trained laboratory personnel, the independent and automated calibration, performance control and error elimination, and minimal maintenance make the GEM Premier 3000 analyzer suitable for work at ICUs.
Acknowledgment
This assessment study was supported by the MD Lab Co., representative of the Instrumentation Laboratory, and University Department of Chemistry, Sestre milosrdnice University Hospital, Zagreb.