Relationship between uric acid, hyperglycemia and hypertriglyceridemia in general population
Giuseppe Lippi
[*]
[1]
Martina Montagnana
[1]
Giovanni Targher
[2]
Gian Luca Salvagno
[1]
Gian Cesare Guidi
[1]
Introduction
Epidemiological studies have shown significant associations between increased serum uric acid concentrations and several components of the metabolic syndrome, such as obesity, type 2 diabetes and hypertriglyceridemia (1,2). Notably, these associations were already present in children and young adolescents (3,4). It was also recently emphasized that elevated serum uric acid levels are strongly associated with the metabolic syndrome, yet are not an independent risk factor for vascular disease in patients with this syndrome (5). Additionally, Liou et al. reported that the presence of metabolic syndrome was not associated with increased circulating uric acid levels (6), whereas Lin et al. did not observe any association between serum uric acid, hyperglycemia and other components of the metabolic syndrome (7). Thus, this study was designed to unveil potential biological relationships between uric acid, fasting plasma glucose (FPG) and triglycerides in the general population.
Materials and methods
Patients
We performed a retrospective analysis on the database of our Laboratory Information System to retrieve results of serum uric acid, FPG and triglyceride tests (the two key biochemical components of the metabolic syndrome according to the ATP III definition) (8), which had been performed on the whole cohort of outpatient adults consecutively referred by general practitioners for routine blood testing in the previous two years (June 2005 – June 2007). Neither inclusion nor exclusion criteria were applied to stratify the entire population of outpatients.
Methods
Venous blood was routinely collected in the morning on fasting subjects. FPG, triglycerides and uric acid concentrations were assayed by standard enzymatic procedures on Roche/Hitachi Modular System (Roche Diagnostics GmbH, Milan, Italy). The upper limit of the reference range for uric acid was 506 μmol/L for males and 416 μmol/L for females.
Statistical analysis
Significance of differences and frequency distribution of values were assessed by the Kruskal-Wallis test (for continuous variables) and the chi-squared test (for categorical variables), respectively. Multivariable linear regression analysis was also preformed but skewed variables were first logarithmically transformed to improve normality. In the fully adjusted multivariable regression model, uric acid was entered as a dependent variable, whereas age, gender, FPG and triglycerides were included as covariates. Statistical analyses were performed using the statistical package SPSS version 12.0 (SPSS, Chicago, IL) and the level of statistical significance was always set at P < 0.05. Data are presented as means (± 95% confidence intervals) or percentages. The study was approved by our departmental ethics committee.
Results
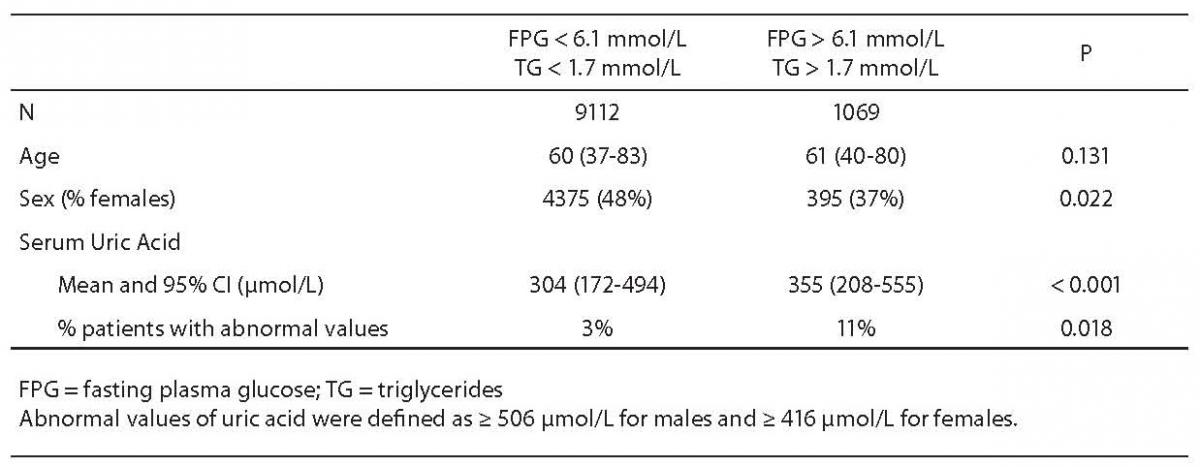
Cumulative results for serum uric acid, FPG and triglyceride levels were retrieved for 10,181 outpatients > 35 years old over the 2-year period. As shown in Table 1, subjects with both FPG and triglyceride values exceeding the thresholds defined by the ATP III criteria were more likely to be male and had a marked increase in serum uric acid levels. Almost identical results were found after stratifying the study population between males (367 μmol/L, 95% CI = 226–579 μmol/L vs 338 μmol/L, 95% CI = 208–512 μmol/L; P < 0.001) and females (334 μmol/L. 95% CI = 201–525 μmol/L vs 270 μmol/L, 95% CI = 155–458 μmol/L; P < 0.001). The differences in serum uric acid levels remained statistically significant even after adjustment for age (data not shown). Accordingly, the frequency of abnormal values of uric acid was higher among subjects with high FPG and triglyceride values, suggestive for diagnosing the metabolic syndrome, than among those with normal FPG and triglyceride values (Table 1). The study subjects with hyperuricemia were also characterized by a significantly higher prevalence of abnormal values of both FPG and triglycerides (32% versus 11%; P < 0.001) as compared to subjects with serum uric acid values within the reference range. In multivariable linear regression analysis, FPG (standardized beta coefficient = 0.520; P < 0.001) and triglycerides (standardized beta coefficient = 0.293; P < 0.001) were independently associated with serum uric acid levels, after adjustment for age and gender.
Table 1. Baseline characteristics of the study participants (N = 10,181) grouped according to the prevalence of abnormal glucose and triglyceride values (as defined by the ATP III criteria) (9).
Discussion
There is debate whether uric acid is simply a marker of cardiovascular disease or it may exert an atherogenic effect independently of other known cardiovascular risk factors. Elevated levels of uric acid correlate with older age, male gender, hyperlipidemia, obesity, insulin resistance and type 2 diabetes (1,2) and accelerate the progression of hypertension-induced end-organ injury (9). Uric acid also activates the complement system inducing the development of oxidative stress and LDL oxidation (10), and exerts proinflammatory effects stimulating human mononuclear cells to produce inflammatory cytokines (9). Finally, uric acid induces systemic endothelial dysfunction, a pathogenetic mechanism in mediating hypertension (11).
The major finding of this study is that hypertriglyceridemic and hyperglycemic adults have increased prevalence rate of elevated serum uric acid levels, and that hypertriglyceridemia and hyperglycemia are the strongest predictors of hyperuricemia in a large sample of the general population. At first glance, these findings could appear unsurprising, given the strong association between serum uric acid levels and insulin resistance and the previous observations of a positive association of serum uric acid levels with hyperglycemia and dyslipidemia (4). However, the underlying pathophysiological mechanisms linking hyperglycemia, hypertriglyceridemia and hyperuricemia are currently unknown. Both the factors that increase serum uric acid synthesis (e.g., an increased activity of the hexose monophosphate shunt and thereby purine biosynthesis) or those that decrease urinary uric acid excretion rate (e.g., an increased tubular reasorption and/or diminished tubular secretion) might be involved. Indeed, it has been shown that patients with insulin resistance or impaired glucose tolerance have reduced values of urinary uric acid clearance (12) and chronically increased extracellular adenosine concentrations, thereby contributing to increasing uric acid synthesis (13).
The strengths and limitations of the present study deserve comment. The biochemical variables (hyperglycemia, hypertriglyceridemia) that typically cluster in the metabolic syndrome were retrieved from a large database of outpatient test results and confirm the previous observation that FPG was significantly and positively associated with the uric acid level (14). However, the cross-sectional design of the study precludes the establishment of causal or temporal relations among these variables, and prospective studies will be required to sort out the time sequence of events. Further, the study population of outpatient adults from laboratory may not be a representative sample of general population and, unfortunately, neither additional clinical information is available on this large cohort of outpatients, nor the effective prevalence of metabolic syndrome as defined by the complete ATP III criteria. Nevertheless, the biological interrelationships observed in this large study population raise the possibility of a potential pathogenetic overlap (or a vicious circle) between hyperuricemia, hypertriglyceridemia and hyperglycemia.
Notes
Potential conflict of interest
None declared
References
1. Saggiani F, Pilati S, Targher G, Branzi P, Muggeo M, Bonora E. Serum uric acid and related factors in 500 hospitalized subjects. Metabolism 1996;45:1557-6.
2. Choi HK, Ford ES. Prevalence of the metabolic syndrome in individuals with hyperuricemia. Am J Med 2007;120:442-7.
3. Bonora E, Targher G, Zenere MB, Saggiani F, Cacciatori V, Tosi F, et al. Relationship of uric acid concentration to cardiovascular risk factors in young men. Role of obesity and central fat distribution. The Verona Young Men Atherosclerosis Risk Factors Study. Int J Obes Relat Metab Disord 1996;20:975-80.
4. Ford ES, Li C, Cook S, Choi HK. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation 2007;115:2526-32.
5. Hjortnaes J, Algra A, Olijhoek J, Huisman M, Jacobs J, van der Graaf Y, Visseren F. Serum uric acid levels and risk for vascular diseases in patients with metabolic syndrome. J Rheumatol 2007 Aug 1; ŠEpub ahead of printĆ.
6. Liou TL, Lin MW, Hsiao LC, Tsai TT, Chan WL, Ho LT, et al. Is hyperuricemia another facet of the metabolic syndrome? J Chin Med Assoc 2006;69:104-9.
7. Lin JD, Chiou WK, Chang HY, Liu FH, Weng HF. Serum uric acid and leptin levels in metabolic syndrome: a quandary over the role of uric acid. Metabolism 2007; 56:751-6.
8. Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults. Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert PANEL on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486–97.
9. Mazzali M, Kanellis J, Han L, Feng L, Xia YY, Chen Q, et al. Hyperuricemia induces a primary arteriolopathy in rats by a blood pressure-independent mechanism. Am J Physiol Renal Physiol 2002;282:F991–7.
10. Leyva F, Anker S, Swan JW, Gotsland EF, Wingrove CS, Chua TP, et al. Serum uric acid as an index of impaired oxidative metabolism in chronic heart failure. Eur Heart J 1997;18:858-65.
11. Johnson RJ, Kang D-J, Feig D, Kivlighn S, Kanellis J, Watanabe S, et al. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension 2003;41:1183-90.
12. Facchini F, Chen YD, Hollenbeck CB, Reaven GM. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA 1991;266:3008-11.
13. Bakker SJ, Gans RO, ter Maaten JC, Teerlink T, Westerhoff HV, Heine RJ. The potential role of adenosine in the pathophysiology of the insulin resistance syndrome. Atherosclerosis 2001;155:283-90.
14. Selby JV, Newman B, King MC, Friedman GD. Environmental and behavioral determinants of fasting plasma glucose in women. A matched co-twin analysis. Am J Epidemiol 1987;125:979-88.