Introduction
Development, course and complications of type 2 diabetes mellitus are closely associated with imbalance of pro- and antioxidative cell impairment and change of redox potential. Oxidative stress in diabetes is the result of both increased production of free radicals and reduced capacity of antioxidative defense (1).
Antioxidative defense of organism which involves enzymatic and nonenzymatic substances maintains the balance by generation of free radicals within homeostasis limits and prevents the expansion of free-radical reaction that may cause tissue damage (2).
Superoxide dismutase (SOD; E.C. 1.15.1.1) catalyzes dismutation of superoxide radicals into hydrogen peroxide and molecular oxygen.
Glutathione peroxidase (GPx; E.C. 1.11.1.9) catalyzes reduction of hydrogen peroxide or organic hydroperoxide into alcohol in the presence of reduced glutathione as an electron donor. Constant regeneration of reduced glutathione is carried out by activation of glutathione reductase (GR; E.C. 1.6.3.2).
Uric acid is an important physiological antioxidant. Action of free radicals makes uric acid to be oxidized into allantoin, while iron and copper ion binding produces stable complexes, which reduces the oxidation potential of these elements as well as propagation of free-radical reactions. In addition, uric acid neutralizes hydroxyl radical and hypochloric acid (3).
In conditions of low partial oxygen pressure, bilirubin acts as a potent “scavenger” of peroxyl radicals (4).
Albumin, ferritin, transferrin, haptoglobin and ceruloplasmin, by the binding of iron or copper ions, significantly decrease the production of free radicals, thus protecting the molecules of free fat acids from peroxidation. On the other hand, albumin may neutralize hypochloric acid as well as peroxyl radicals (5-7).
The objectiveof our study was to determine the interrelations between the “first”, non-enzymatic (uric acid, albumin, total proteins, bilirubin, haptoglobin and TAS - total antixidant status) and the “second”, enzymatic (SOD, GPx, GR) line of antioxidant defense in patients with type 2 diabetes mellitus (DM) and manifested cardiovascular complications (CVC) (DM + CVC). The second aim was to determine the relations between the pro-oxidant (lipid status) and antioxidant parameters in studied patients, comparing them to healthy control subjects and complication-free diabetics in order to analyze the impact of cardiovascular complications on the antioxidant defense system.
Materials and methods
Subjects
Our case-control study included 117 type 2 diabetic patients treated at the Institute of Endocrinology, Diabetes and Metabolic Disorders, Clinical Center of Serbia, Belgrade (62 males and 55 females) and 42 healthy subjects (33 women and 9 men), who comprised the control group. The diagnosis of diabetes was made on the basis of patients’ clinical features and laboratory findings: fasting glycemia over 7 mmol/L in two subsequent measurings, and/or higher than 11.1 mmol/L two hours after 75 g oral glucose load. The study also included type 2 diabetic patients who were treated in the afore mentioned Institute and were on oral antidiabetic or insulin therapy longer than 1 year. Criteria for hypertension were: systolic blood pressure over 140 mmHg and diastolic blood pressure over 90 mmHg; and/or the information that patients were on some antihypertensive treatment longer than 1 year.
Healthy subjects were recruited from the employees of the Institute of Medical Biochemistry, Clinical Center of Serbia, Belgrade, who were healthy at the time of study, without any signs of acute or chronic conditions and who did not take any additional antioxidants in their food. They were selected among those individuals who went through regular medical check-up and whose laboratory findings showed the absence of diabetes, hypertension, coronary disease or coronary disorder.
All subjects gave their informed consent on participation in the study, and the local ethic committee approved this study.
Samples
The blood samples for analysis were taken after 12–14-hour overnight fast. All laboratory tests were done immediately after sampling.
Antioxidant parameters SOD, GPx, GR and TAS, were determined by commercial tests (Randox Ltd., UK), based on spectrophotometer methods by Goldstein (for SOD) (8), Paglia and Valentine (for GPx) (9), Miller (for TAS) (10) and Goldberg (for GR) (11), while other non-enzymatic and lipid parameters were determined by standard laboratory tests.
SOD was determined in blood hemolysate obtained by washing of erythrocytes 4 times with 3 mL of NaCl (154 mmol/L) and finally by lysing of washed erythrocytes with cold deionized water, followed by a period of 15 minutes at +4 oC to complete hemolysis.
Glutathione peroxidase was determined in the whole blood sample which was, just before determination, diluted 41 fold by gradual addition of diluent (supplied in the test kit) and double-concentrated Drabkin’s reagent.
Glutathione peroxidase and TAS were determined in plasma that was obtained by centrifugation of Li-heparinized blood 10 min/3000 rpm.
Statistical analysis
Results were presented as mean ± SD for continuous normally distributed variables. Statistical analysis of differences between all groups was performed using ANOVA test. Spearman’s correlation test was used to define correlations of individual parameters between and within groups. Statistical analyses were performed using SPSS v10.0 (SPSS Inc. Chicago IL) statistical software. All statistical tests were two-tailed. P values ≤ 0.05 were considered statistically significant.
Results
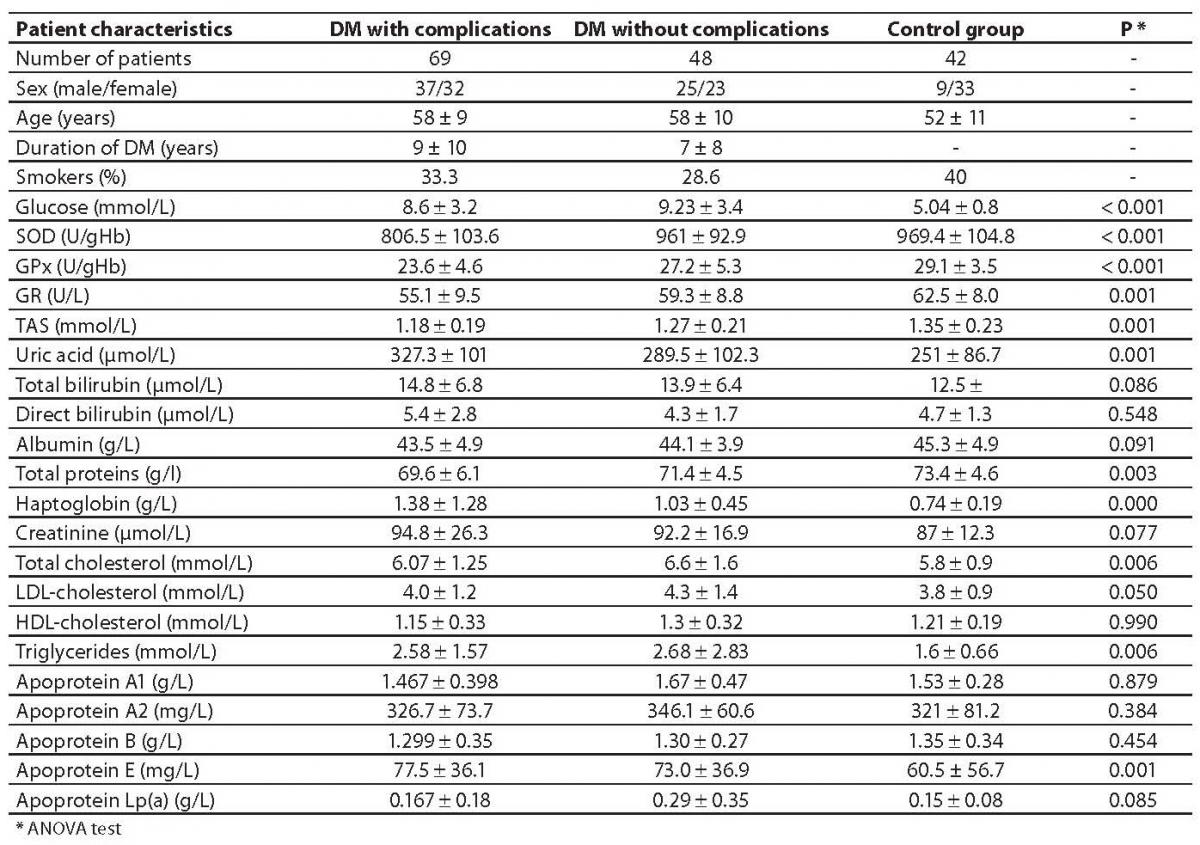
The values of tested parameters and general information on subjects are presented in Table 1.
Table 1. Demographic characteristics and measured biochemical parameters of the groups under observation.
Of a total number of studied diabetic patients, 48 subjects, (23 females and 25 males), aged 58 10 years, had type 2 diabetes without complications (DM), while 69 subjects, (32 females and 37 males), aged 57 9 years, were diagnosed with cardiovascular complications (DM + CVC) according to the World Health Organization criteria such as coronary artery disease (CAD), hypertension (HTA) and a personal history of acute myocardial infarction (AMI) in the last 8 years. Duration of diabetes in the DM + CVC group was 9 10 years, and complications lasted from 1 to 32 years, while in the group DM without complications, diabetes lasted 7 8 years.
In the group DM + CVC, 32 cases of coronary disease with hypertension (46.4%) (CAD + HTA), 17 patients without hypertension (CAD) (24.6%), 7 patients (10.2%) with coronary disease and personal history of myocardial infarction (CAD + AMI) were documented, and 13 patients (18.8%) had all three complications (CAD + AMI + HTA).
In the DM + CVC group, the therapy involved dietary regime in 29 patients (42%), and 30 patients (43.5%) were administered oral hypoglycemic drugs, while 10 patients (14.5%) received insulin combined with oral hypoglycemics. In the group without complications, 21 patients (43.75%) had only dietary regime, and the rest (56.25%) were administered oral hypoglycemic drugs.
The obtained SOD, GPx and GR values were significantly lower in type 2 diabetic patients with cardiovascular complications (P < 0.001) compared to healthy control subjects. Significant difference was found between two subgroups of type 2 diabetic patients. The enzymatic antioxidant parameters were much lower in the subgroup of type 2 diabetics with cardiovascular complications compared to the subgroup of complication-free diabetic patients (P < 0.001 for SOD and GPx and P = 0.025 for GR).
The values of enzymatic antioxidant parameters were different from those for non-enzymatic antioxidant substances. Uric acid values were significantly higher in DM + CVC in relation to the controls (P < 0.001), but not in relation to DM without complications (Table 1). Total bilirubin was also higher in the group DM + CVC in relation to control group (P = 0.05), but not in relation to DM without complications. Direct bilirubin value was not significantly different in relation to control group, but significant difference was found between the groups DM + CVC and DM with no complications. Albumin levels were not significantly different between these groups, while haptoglobin values were significantly higher in both studied groups in relation to the control group (P < 0.001 and P = 0.001, respectively).
A series of significant correlations between enzymatic and non-enzymatic parameters was obtained in all three groups of subjects. In DM + CVC, weak but significant correlations were obtained between SOD and haptoglobin values (R = 0.35, P = 0.049); SOD and total proteins (R = 0.29, P = 0.049) as well as between TAS and uric acid (R = 0.35, P < 0.001), while weak significant negative correlations were found between GPx and uric acid (R = –0.35, P = 0.009) and GPx and total bilirubin (R = –0.40, P = 0.012).
In the group DM without complications, TAS values correlated moderately with bilirubin (R = 0.54, P < 0.001), and GPx values correlated weakly with total proteins (R = 0.395, P = 0.015).
Total cholesterol concentrations were similar in both groups of affected subjects, and significant difference was obtained in comparison to control group (P = 0.040 and P = 0.001, respectively). Triglyceride values were significantly higher in both groups in relation to controls, while HDL and LDL cholesterol levels were similar in all three groups of subjects. Values of apoproteins apo A1, apo A2 and apo B were similar in all three groups of subjects, while apo E concentrations were significantly lower in the control group than in diabetics with complications (P = 0.045).
TAS levels weakly correlated with triglycerides (R = 0.32, P = 0.016) while GPx and GR activities correlated positively with HDL cholesterol in both diabetic groups (R = 0.457, P = 0.007 and R = 0.466, P = 0.001 for DM without and with complications respectively). Weak but positive correlation between GR and apoprotein A1 (R = 0.289; P = 0.040) was found in the DM + CVC group.
In healthy controls, statistically significant but weak correlations were found between SOD values and total proteins (R = 0.395, P = 0.001), albumin (R = 0.326, P = 0.039) and uric acid (R = 0.378, P = 0.014), while there was a weak inverse correlation between GPx and total proteins and albumins (R = –0.329, P = 0.037 and R = –0.369, P = 0.019, respectively). Glutathione peroxidase correlated weakly with LDL-cholesterol (R = 0.376, P = 0.028) as well as with apo A2 and apo B (R = 0.477; P = 0.005; and R = 0.381, P = 0.029, respectively).
It is worth mentioning that TAS and GR values were in weak positive correlation with glucose concentration in healthy controls (R = 0.413, P = 0.006, and R = 0.304, P = 0.049, respectively), which suggested that the increase of serum glucose concentration in healthy controls was associated with higher activity of glutathione reductase and higher concentrations of some non-enzymatic antioxidants.
In diabetics without complications, weak positive correlation between GPx and glucose concentration was also found, as well as between SOD and glucose (R = 0.384 for P = 0.049, and R = 0.375, P = 0.050, respectively), which also indicated the association of higher level of antioxidative defense with higher glucose concentrations in diabetics who had not developed complications.
Spearman’s correlation coefficient disclosed weak negative but significant correlation between GPx and glucose values (R = –0.382, P = 0.049) in DM group with CD and HTA, and also in the DM group with CD and AMI (R = –0.860, P = 0.041); while in the DM group with all three types of complications, significant moderate negative correlation was found between SOD and glucose levels (R = –0.590, P = 0.035).
In healthy controls, SOD correlated moderately with TAS (R = 0.62; P < 0.001), and moderate inverse correlation was observed between SOD and GR (R = –0.58; P < 0.001), whereas GR and TAS were in weak negative correlation (R = –0.358; P = 0.027).
In diabetics with complications, the antioxidative parameters were in mutual positive correlation: we observed weak correlation between SOD and GPx (R = 0.289; P = 0.028) and SOD and GR (R = 0.259; P = 0.045).
Discussion
This was the first study to compare the “first” and the “second” line of antioxidant defense in type 2 diabetic patients, and their relations to pro-oxidant plasma substances such as lipid status parameters, in order to analyze the association of manifested cardiovascular complications and antioxidant defense system.
On the basis of the obtained results, it may be concluded that enzymatic antioxidant defense of type 2 diabetic patients was significantly reduced in comparison with healthy controls (P < 0.001). More profound reduction of enzymatic antioxidant defense was observed in diabetics with cardiovascular complications than in diabetics without complications (P < 0.001).
There is a large number of compounds in our body acting as antioxidants. Many of them are determined on regular basis in our everyday practice such as uric acid, bilirubin, albumin, ferritin, transferrin, ceruloplasmin, haptoglobin, etc. In cases of imbalance of oxidants and antioxidants, organism endeavors, by increased synthesis of some substances, to compensate for lower activity of some enzymes and thus maintains the balance.
Non-enzymatic antioxidant defense was increased in our study subjects, which was confirmed by correlations found between certain non-enzymatic antioxidants and the activity of antioxidant enzymes (GPx and uric acid, GPx and bilirubin, TAS and GR, TAS and GPx).
TAS was in weak positive correlation with values of uric acid and bilirubin, which was consistent with the fact that total antioxidant status (TAS) is a sum determination of all non-enzymatic antioxidants that may counteract hydrogen peroxide in plasma.
There was a huge difference in the extent of antioxidant defense between diabetics with and those without cardiovascular complications, which indicated the significant effect of type and duration of cardiovascular complications on antioxidant defense. In diabetics with cardiovascular complications, enzymatic antioxidants were in positive correlation with each other, while they correlated negatively with non-enzymatic antioxidants (TAS).
In healthy subjects SOD correlated weakly with some non-enzymatic antioxidants such as albumin, total proteins and uric acid, suggesting the possible synergistic effect of the “first” and the “second” line of antioxidant defense. In the same group, GPx correlated inversely with total proteins and albumins, but such correlation was not observed in the group of type 2 diabetics with cardiovascular complications. In the group of patients suffering from type 2 diabetes with cardiovascular complications, GPx correlated negatively with uric acid and total bilirubin, thus indicating the possible association of these parameters with the development of cardiovascular complications in diabetics.
Positive correlations between GPx and LDL-cholesterol in healthy subjects could indicate possible association between high lipid concentrations leading to accelerated lipid peroxidation and potentially increased reduction of organic hydroperoxides as a consequence of increased activity of GPx. No such correlation was found in both groups of diabetic patients, while other significant correlations between GPx and HDL-cholesterol DM + CVC and GR and HDL-cholesterol DM) were found.
In the control group (physiological conditions), the increase of glucose concentration was associated with higher GR activity and TAS levels, whereas in diabetics without complications increased glucose level was associated with higher activity of GPx and SOD. Such correlation was not found in the DM + CVC group. On the contrary, correlation between glucose and SOD was negative in the group of type 2 diabetics with severe cardiovascular complications (CAD + HTA + AMI). In two of our previous studies, we observed that higher glucose concentration was associated with lower GPx activity in diabetic subgroups who had coronary disease with hypertension and AMI, respectively (12,13). Such negative response of antioxidative defense system may be related to the effect of protein glycosylation and impact of oxidative stress on reduced catalytic SOD and GPx activity, all of which contributed to impaired total antioxidative defense of diabetics with cardiovascular complications. Thus changed values of tested parameters and diversity of correlations suggest significantly modified antioxidant defense of diabetics, and indicate different degrees of imbalance not only between oxidants and antioxidants but also between the “pool” of non-enzymatic substances and the activity of enzymatic antioxidants that largely depend upon the presence, type and duration of cardiovascular complications.
Other authors who studied this subject have obtained similar results. Reduced activities of SOD, catalase and ceruloplasmin were also obtained by Abou Seif and his co-workers in type 2 diabetics (14), with increased concentrations of lipid parameters such as cholesterol, triglycerides, LDL-cholesterol, lowered values of HDL-cholesterol and increased concentrations of lipid peroxidation and glycosylation products. Everekliouglu (15) showed that diabetics with macular degeneration had lower activity of SOD, GPx and higher concentrations of MDA and NO in relation to healthy subjects. Patients with longer maculopathy had larger reduction of SOD and GPx in relation to those in the early phase of maculopathy. Martin-Gallan (16) proved that young diabetics with newly detected microangiopathy had significantly reduced values of GPx, reduced glutathione and β-carotene in relation to the controls, but not in relation to diabetics without microangiopathy; in addition, SOD values were significantly higher in both groups of subjects regardless of the presence or absence of microangiopathy. Valabhji and his co-workers observed lower TAS values in type 1 DM in relation to the controls, and those values were in negative correlation with HbA1c values (P = 0.0026), duration of diabetes and aging, particularly in men (17). Older patients with type 1 DM who manifested higher degree of arterial calcification, had higher values of blood pressure, longer duration of diabetic disorder, higher concentrations of serum cholesterol and creatinine and lower values of TAS in comparison with patients with minor arterial calcification. Ruiz (18) and his colleagues found that insulin-dependent diabetics had significantly lower GPx activity and that its reduction directly depended upon the degree of metabolic control. The same author verified that there was a significant correlation of non-enzymatic antioxidants and total cholesterol levels, lipid hydroperoxide, triglyceride and HBA1c concentrations. Nojiri et al (19) demonstrated that TAS values and the concentrations of retinal, albumin, total proteins and HDL-cholesterol were significantly lower in patients with coronary artery disease compared to control subjects. TAS values correlated positively with uric acid and negatively with the number of diseased vessels. Their study demonstrated the association of antioxidant parameters with atherosclerosis progression, but failed to confirm antioxidants as an independent risk factor of CAD event.
In our study, we illustrated the association of some enzymatic antioxidants both with total non-enzymatic antioxidant activity of plasma and individual non-enzymatic antioxidant substances. We chose those substances with antioxidant activity which are most usually determined in current laboratory practice. Besides these substances with antioxidant effect, others (i.e. glutathione, α-tocopherol, zeaxantine, lycopene, ubiquinol and others) also represent the total antioxidant capacity of plasma; they were not included, which is a possible limitation to this study.
Conclusion
In summary, elevated oxidative stress and oxidative tissue damage are common end points of chronic diseases such as atherosclerosis and diabetes. There is considerable evidence that many biochemical pathways adversely affected by hyperglycemia and other substances that are found at elevated levels in diabetic patients are associated with the generation of reactive oxygen species, ultimately leading to increased oxidative stress in a variety of tissues. Diabetic patients with vascular complications may have a defective cellular antioxidant response against the oxidative stress generated by hyperglycemia. Such view encourages the idea that antioxidant therapy of these patients may be of great interest. Thus, further investigations of therapeutic strategies to prevent or delay the progression of diabetic vascular complications are needed.
Acknowledgments
The Ministry of Science and Environmental Protection of Serbia supported this study,on the basis of contract No 145010.
Notes
Potential conflict of interest
None declared
References
1. Aronson D, Rayfield EJ. How hyperglycemia promotes atherosclerosis: molecular mechanisms. Cardiovascular Diabetology 2002;1:1.
2. Korać B, Buzadžić B. Oxidative stress and antioxidative defense in the skin of rats with thermal injury. Jugoslov Med Biochem 2003;22:33-9.
3. Davies KJA, Sevanian A, Muakkassah-Kelly SF, Hochstein P. Uric acid-iron complexes. A new aspect of the antioxidant function of uric acid. Biochem J 1986;235:745-54.
4. Stocker R, Glazer AN, Ames BN. Antioxidant activity of albumin-bound bilirubin. Proc Natl Acad Sci USA 1987;84:5918-22.
5. Halliwell B, Gutteridge JM. Albumin- an important extracellular antioxidant? Biochem Pharmacol 1988;37:569-71.
6. Meira-Melamed F, Lache O, Enav IB, Szafranek T, Levy NS, Ricklis RM, Levy AP. Structure-function analysis of the antioxidant properties of haptoglobin. Blood 2001;98(13):3693-8.
7. De Jong G, van Dijk JP, van Eijk HG. The biology of transferrin. Clin Chim Acta 1990;190:1-46.
8. Goldstein S, Michel C, Boors A, Saran M, Czapsky G. A critical re-evaluation of some assay methods for superoxide dismutase activity. Free Radical Biol Med 1988;4:295-303.
9. Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterisation of glutathione peroxidase. J Lab Clin Med 1967;70:158-63.
10. Miller NJ, Rice-Evans C, Davies MJ, Gopinathan V, Milner A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin Sci 1993;84:407-12
11. Goldberg DM, Spooner RJ. In: Bergmeyer HU, editor. Methods of enzymatic analysis. 3rd ed. New York: Academic Press 1974;258-65.
12. Čolak E, Majkić-Singh N, Stanković S, Djordjević BP, Srećković-Dimitrijević V, Lalić K, Lalić N. The effect of hyperglycemia on the values of antioxidative parameters in type 2 diabetic patients with cardiovascular complications. Jugoslov Med Biohem 2006;25(2):173-9.
13. Čolak E, Majkić-Singh N, Stanković S, Srećković-Dimitrijević V, Djordjević PB, Lalić K, Lalić N. Parameters of antioxidative defense in type 2 diabetic patients with cardiovascular complications. Ann Med 2005;37:613-20.
14. Abou-Seif MA, Youssef AA. Evaluation of some biochemical changes in diabetic patients. Clin Chim Acta 2004,16; 346(2):161-70.
15. Evereklioglu C, Er H, Doganay S, Cekmen M, Turkoz Y, Otlu B, Ozerol E. Nitric oxide and lipid peroxidation are increased and associated with decreased antioxidant enzyme activities in patients with age-related macular degeneration. Doc Ophtalmol 2003;106(2):129-36.
16. Martin-Gallan P, Carrascosa A, Gussinye M, Dominguez C. Biomarkers of diabetes-associated oxidative stress and antioxidant status in young diabetic patients with or without subclinical complications. Free Radic Biol Med 2003;15;34(12):1563-74.
17. Valabhji J, McCool AJ, Richmond W, Shatter M, Rubens MB, Elkeles RS. Total antioxidant status and coronary artery calcification in type 1 diabetes. Diabetes Care 2001;24:1608-13.
18. Ruiz C, Alegria A, Barbera R, Ferre R, Lagarda MJ. Lipid peroxidation and antioxidant enzyme activities in patients with type 1 diabetes mellitus. Scan J Clin Lab Invest 1999:59(2):99-105.
19. Nojiri S, Daida H, Mokuno H, Iwama Y, Mae K, Ushio F, Ueki T. Association of serum antioxidant capacity with coronary artery disease in middle-aged men. Jpn Heart J 2001;42(6):677-90.