Development of a preanalytical errors recording software
Giuseppe Lippi
[*]
[1]
Patrizia Bonelli
[1]
Rossana Rossi
[1]
Mirco Bardi
[1]
Rosalia Aloe
[1]
Alberta Caleffi
[1]
Enrichetta Bonilauri
[1]
Introduction
Ever since, the contribution of laboratory diagnostics is integral to the clinical decision making, providing a substantial contribution to screen, diagnose, monitor and follow-up of the patients. In this scenario, quality and safety in laboratory testing are essential to furthering the goal of high-quality and safe healthcare (1). Although a significant decrease of the error rate in clinical laboratories has occurred over the past decades, currently available evidence demonstrates that the pre- and post-analytical activities of the total testing process are more error-prone than the analytical phase (2). Preanalytical variability, in particular, is a major source of concern because it accounts for up to 70% of the errors occurring within the entire diagnostic process, it can adversely impact on test results, patient’s health and laboratory organization (3-5). From a practical point of view, error reduction can be achieved through a total quality management system, encompassing an integrated approach and a multifaceted strategy for process and risk analysis funded on reliable quality indicators for both the analytical and extra-analytical phases of testing (6), and a suitable, effective and multifaceted approach for prevention, identification, recording, analysis and management of errors (7,8). Information technology provides a valuable aid to laboratory professionals for identifying, controlling and decreasing the error rate in the total testing process, including the preanalytical and postanalytical phase (9). As such, implementation of a software for systematical recording of errors, especially for those laboratories that do not have a laboratory information system (LIS), would carry great advantages over classical paper datasheets, including the possibility to produce proper statistics and reports on preanalytical errors. The aim of this article is to describe the software developed for the recording of preanalytical errors in our laboratory.
Materials and methods
Following the consensus recommendations of the Italian Inter-society SIBioC-SIMeL-CISMEL (Society of Clinical Biochemistry and Clinical Molecular Biology - Italian Society of Laboratory Medicine - Italian Committee for Standardization of Hematological and Laboratory Methods) Study Group on Extra-analytical Variability for detection and management of unsuitable samples in clinical laboratories (10), we have developed an error recording software based on Microsoft Access for facilitating and standardizing the practice of recording unsuitable samples in our laboratory. Microsoft Access was chosen for several reasons. First, it is widely available on most personal computers and laptops, then it is relatively easy to use and does not require peculiar informatics skill to be programmed and, finally, it allows exportation or download of data on several statistics platforms (e.g., Excel).
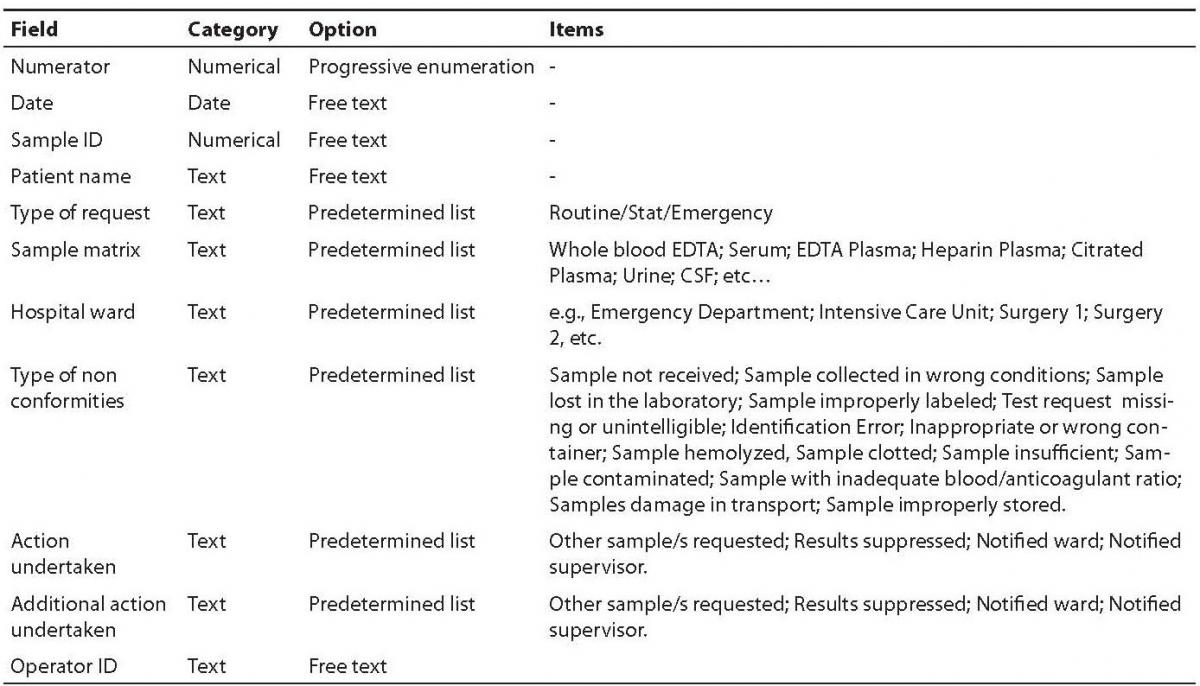
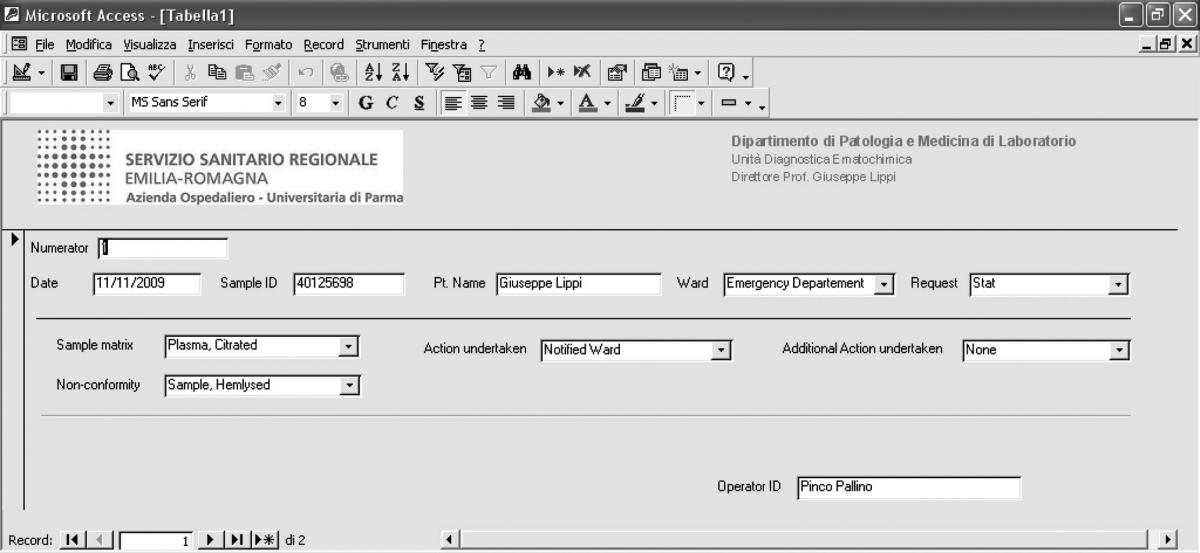
The main fields included in the software comprehend a numerator for progressive enumeration of the samples, the date of receipt of the specimen, the Sample ID, the patient’s name, the type of request (stat/routine), the referring ward, sample matrix (e.g., whole blood EDTA; serum; EDTA plasma; citrated plasma, urine, etc.), the type of non-conformity (e.g., sample hemolyzed, sample clotted, etc.), the action undertaken to solve the problem (e.g., request additional specimen, results suppressed, etc), a second field for possible additional actions undertaken (e.g., other sample/s requested; results suppressed; notified ward), and the operator ID (Table 1). The definitive graphical layout is shown in figure 1. The database is stored on a common repository in our laboratory information system, so that it can be accessed by any computer in the laboratory, allowing continuous and standardized input of the data.
Table 1. Fields and composition of the database
Figure 1. Graphical layout of the preanalytical errors recording software
Results and discussion
It is generally assumed that we need to improve and internalize the culture of safety throughout all levels of healthcare, for being proactive and reducing the large burden of medical and diagnostic errors. As quality and safety movements gallop along, the need to reduce laboratory errors spreads contextually. The growing awareness that extra-analytical activities of the total testing process are more vulnerable to errors than the analytical ones has led to important initiatives for reducing the uncertainty in this area (11), such as the focus currently being placed on collecting information on self reported routines and procedures for the extra-analytical phases of laboratory practice in some European countries (12,13).
As such, the systematic identification and recording of preanalytical errors is essential, since it would help streamline specific responsibilities, produce useful information on the local development of preanalytical processes, on wards or departments more prone to errors, troubleshoot faults, thus providing the ideal root for eliminating bottlenecks and flaws, and redesigning the structure of the entire system of diagnostic testing more safely and efficiently. Process-supporting information technology has been heralded as an effective building block to improve the quality and safety of laboratory diagnostics (14). The implementation of a software for recording preanalytical errors in the daily practice would thereby grant major benefits, in that it would enable to harmonize procedures of incident reporting and facilitate digital recording (15). The additional advantages of the computerized system that we have developed encompasses easy of use (the software is based on Microsoft Access, which is integrated in the software of most office computers, laptops and even smart phones), elimination of handwritten reports, possible translation into different languages, inclusion of a variety of validated measures of laboratory performance such as the quality indicators proposed by the Working Group, “Laboratory Errors and Patient Safety (WG-LEPS) of the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) (16), handily customization to suit local needs as well as standardization of formats among different laboratories which would make both comparison and benchmarking more viable. The database can also be linked to or imported in an Excel worksheet, thereby carrying additional advantages over traditional paper datasheets, that are the possibility to perform comprehensive statistical analyses on errors type and frequency including a variety of data (e.g., enumeration of the samples, the date of receipt of the specimen, the Sample ID, the patient’s name, the type of request - stat/routine -, the referring ward, the sample matrix), improved data searching and processing, production of better statistical reports, and to monitor the effectiveness of changes eventually introduced throughout the different activities of the total testing process. All these aspects might trustworthily support the ongoing efforts to improve the quality of laboratory diagnostics, especially the quality of the extra-analytical activities (17).
The system has been established in our lab very recently, so no statistically significant information could be collected thus far. As such, the next necessary step is to benchmark this analysis with the previous practice that was reporting errors on traditional datasheets. As yet, however, we can comment that practice of systematical error reporting in our laboratory was substantially increased, greatly eased and standardized among the different sections and professionals. Although some laboratory information management systems (LIMS) e.g. Swisslab (F. Hoffmann-La Roche, Basel, Switzerland), Starlims (STARLIMS Corporation Hollywood, FL, USA) have already the capability to monitor and record sample quality and to export these data to standard software for comprehensive statistical analysis, the software we have developed may be advantageous for those clinical laboratories that do not have a LIS or LIMS with error-recording options, or which want to remain on the old LIS without moving to a more expensive LIMS.
Notes
Potential conflict of interest
None declared.
References
1. Plebani M, Lippi G. To err is human. To misdiagnose might be deadly. Clin Biochem. 2009 Jul 14. [Epub ahead of print].
2. Plebani M. Exploring the iceberg of errors in laboratory medicine. Clin Chim Acta 2009;404:16-23.
3. Lippi G, Guidi GC, Mattiuzzi C, Plebani M. Preanalytical variability: the dark side of the moon in laboratory testing. Clin Chem Lab Med 2006;44:358-65.
4. Plebani M. Errors in laboratory medicine and patient safety: the road ahead. Clin Chem Lab Med 2007;45:700-7.
5. Favaloro EJ, Lippi G, Adcock DM. Preanalytical and postanalytical variables: the leading causes of diagnostic error in hemostasis? Semin Thromb Hemost 2008;34:612-34.
6. Simundic AM, Topic E. Quality indicators. Biochem Med 2008;18:311-19.
7. Lippi G, Guidi GC. Risk management in the preanalytical phase of laboratory testing. Clin Chem Lab Med 2007;45:720-7.
8. Lippi G. Governance of preanalytical variability: travelling the right path to the bright side of the moon? Clin Chim Acta 2009;404:32-6.
9. Guidi GC, Poli G, Bassi A, Giobelli L, Benetollo PP, Lippi G. Development and implementation of an automatic system for verification, validation and delivery of laboratory test results. Clin Chem Lab Med 2009;47:1355-60.
10. Lippi G, Banfi G, Buttarello M, Ceriotti F, Daves M, Dolci A, et al. Recommendations for detection and management of unsuitable samples in clinical laboratories. Clin Chem Lab Med 2007;45:728-36.
11. Lippi G, Simundic AM. Total quality in laboratory diagnostics. It’s time to think outside the box. Biochem Med 2010;20:5-8.
12. Self reported routines and procedures for the extra-analytical phase of laboratory work practice in Croatia – cross-sectional survey study. Biochem Med 2010;20:64-74.
13. Lippi G, Montagnana M, Giavarina D. National survey on the pre-analytical variability in a representative cohort of Italian laboratories. Clin Chem Lab Med 2006:44;1491-4.
14. Lippi G, Plebani M. Informatics aids to reduce failure rates in notification of abnormal outpatient test results. Arch Intern Med 2009;169:1815.
15. Lippi G, Mattiuzzi C, Plebani M. Event reporting in laboratory medicine. Is there something we are missing? MLO Med Lab Obs 2009;41:23.
16. Sciacovelli L, Plebani M. The IFCC Working Group on laboratory errors and patient safety. Clin Chim Acta 2009;404:79-85.
17. Plebani M. Laboratory errors: How to improve pre- and post-analytical phases? Biochemia Medica 2007;17:5-9.