Introduction
Bronchoconstriction is the constriction of the airways due to the tightening of surrounding smooth muscle, and clinical consequences of such state are coughing, wheezing, and shortness of breath (1). It can be caused by different clinical conditions, i.e. chronic obstructive lung diseases (asthma, chronic obstructive pulmonary disease – COPD, bronchiectasis), viral infections, interstitial lung disease, extrathoracic lesions (pharinx, larynx, and upper trachea) and intrathoracic lesions (lower trachea). In childhood, bronchoobstruction is mostly caused by respiratory viral infections and asthma. In sensitive individuals, the inflammation is usually induced by causal allergens leading to the symptoms of asthma (bronchoconstriction, cough, chest tightness). Additionally, respiratory infections, especially respiratory viral infections (including rhinoviruses, influenza, parainfluence, respiratory syncytial viruses, coronaviruses, adenovirus) can trigger asthmatic symptoms, such as wheezing and chest tightness (2).
Determination of serum eosinophil cationic protein (3), and recently, determination of serum CRP (using high sensitive method) can be used to monitor inflammation in patients with asthma (4). However, importance of CRP, as a marker of chronic, latent inflammation is not enough tested.
Both, investigations in vitro (5) and in vivo (6) demonstrated that magnesium acts on bronchial smooth muscle relaxation. It is known that many metabolic functions of magnesium and calcium are tightly inter-related. As intracellular cation, magnesium acts as a calcium channel blocker (7). This is consistent with the fact that magnesium also acts as a calcium antagonist. Calcium is known to act as a bronchoconstrictor. Additionally, magnesium is also involved in the processes of inflammation (8) and oxidative stress (9). Hypomagnesaemia may result in reduced pulmonary function, especially in patients with asthma (10). In previous investigations we have point out at the significant redistribution of magnesium between plasma and leukocytes during acute asthma in children (11). Patients with acute asthma (12) and even those with chronic asthma (13) have increased concentration of CRP.
The aim of this study was to find out possible changes in serum concentration of magnesium and calcium (as participants in bronchoconstriction), and concentration of CRP (as a marker of inflammation) in children with moderate and severe bronchoconstriction, caused by viral respiratory infections. CRP, magnesium and calcium concentrations were determined on hospital admission and at discharge from the hospital, i.e. before and after administration of salbutamol (short-acting β2-adrenergic receptor agonist, bronchoconstriction reliever).
Materials and methods
Patients
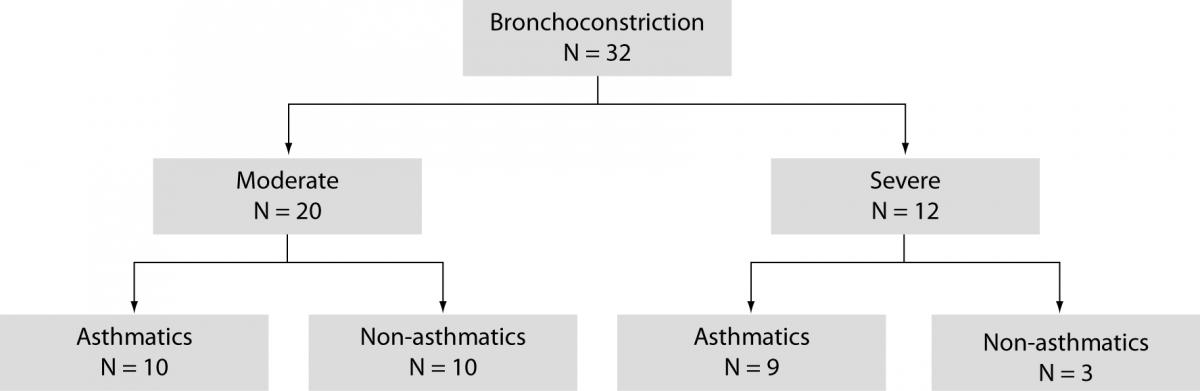
The study included 63 children, aged 1–16 years (6 4 years), divided into two groups: children with acute bronchoconstriction caused by a viral respiratory infection (N = 32), and clinically healthy children referred for systematic medical check-up as a control group (N = 31). All patients were referred from primary health care offices to hospital between December 2008 and January 2009. Based on GINA guidelines (1) moderate bronchoconstriction was confirmed in 20 children, and severe bronchoconstriction in 12 children (Figure 1).
Figure 1. Subgroups of children with bronchoconstriction.
Previously, in 13 children moderate permanent asthma was diagnosed according to the criteria recommended by the PRACTALL (14) and GINA 2006 (1). During respiratory syncytial virus infection (confirmed in nasopharyngeal wash specimens), moderate bronchoconstriction was confirmed in 10 children, and severe bronchoconstriction in 9 children (Figure 1). Allergic disease was not evidenced in other 13 children.
Salbutamol solution (150–200 μg in a single dose) with an ECONOneb nebulizer System (Medix Limited; Lutterworth, UK) was administered at different time intervals, according to the severity of bronchoconstriction. Diagnostic work-up and therapy were performed according to the standardized procedure, in line with ethical principles, Helsinki Declaration on Human Rights from 1975 and Seoul amendments from 2008. Study approval was obtained from the Hospital Ethics Committee. An informed consent in writing was obtained from the parents. Blood sampling was done on admission (Day 1) and the third day of salbutamol administration (Day 3).
Methods
The serum concentrations of magnesium and calcium were determined using standardized spectrophotometric methods, and CRP (high sensitivity C-reactive protein) concentration was determined by immunoturbidimetric method on latex particles (15), on an Olympus AU 400 selective autoanalyzer (Olympus, Tokyo, Japan). Reagents from the same manufacturer were used. Complete blood cell count was analyzed using Sysmex XT-1800i blood cell counter (Sysmex. Corporation, Kobe Hyogo, Japan).
Statistical analysis
The variables were described as mean and standard deviation (x¯ ± SD) if they had normal distribution, or median and interquartile range (IQR) if not. Normality was tested using D’Agostino-Pearson test. Student’s t-test (for normal distribution) and Mann-Whitney test (for asymmetric distribution) were used for comparison of independent samples, while variance ratio, F-test was used for comparison of dependent variables (16). Values of P < 0.05 were considered statistically significant. Correlation of the study variables was expressed by coefficient of correlation and Spearman’s coefficient of rank correlation, respectively (r). Data processing was performed using MedCalc software (Medisoftware, Mariakerke, Belgium).
Results
Inhalation of salbutamol led to a relief of dyspnea within 24 to 36 hours. Also, signs and symptoms of viral infection have gradually relieved.
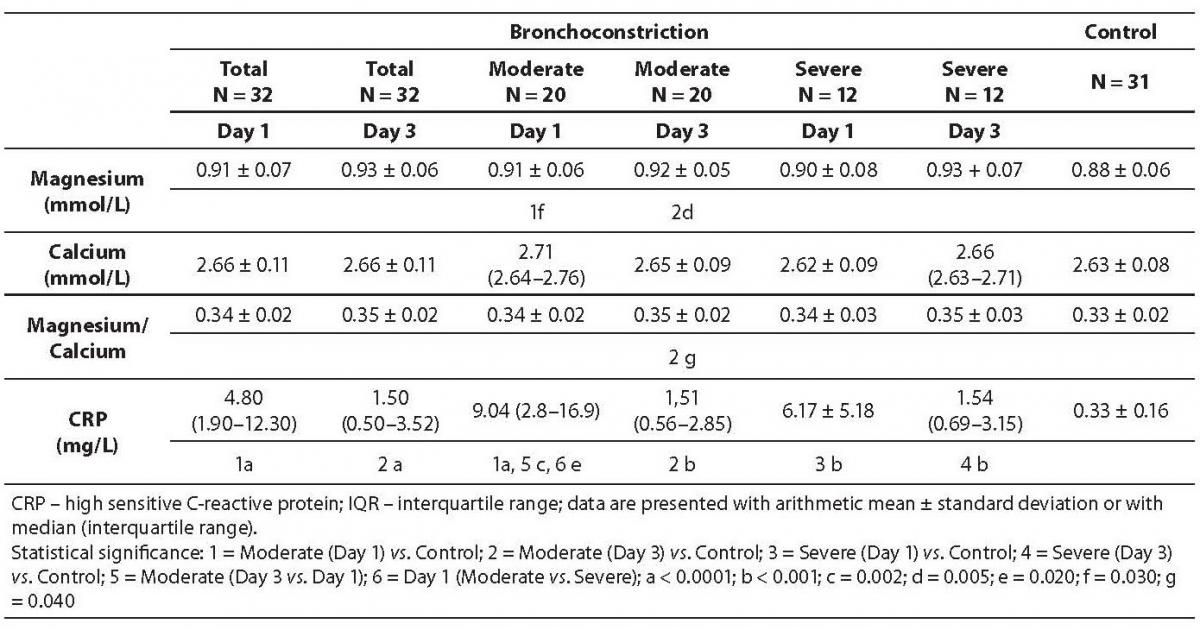
During the follow-up period, concentration of magnesium was statistically significantly higher than in control group of children (Table 1). Statistical differences between magnesium concentration between 1st and 3rd day were not significant, neither in children with moderate nor severe bronchoconstriction. There was no statistically significant difference in plasma calcium concentrations between investigated groups of children. Magnesium to calcium ratio was significantly higher on 3rd day of therapy in comparison with the group of healthy children (P = 0.04).
Table 1. Values of magnesium, calcium, magnesium to calcium ratio and CRP, in 32 children with bronchoconstriction before (day 1) and after therapy with salbutamol.
After administration of salbutamol, in the majority of patients with severe bronchoobstruction (10/12), concentration of magnesium increased, and calcium decreased, resulting in increased values of magnesium to calcium ratio in most children. In the subgroup of children with moderate broncoobstruction, concentration of magnesium increased in 5/10 of children, decreased in 10/20 of children, and remained unchanged in 5/20 of children. Simultaneously, concentration of calcium increased in 8/20, decreased in 6/20 of children, and remained unchanged in 6/20 of children. Magnesium to calcium ratio decreased in 11/20 of children, increased in 1/20 of children and remained unchanged in 8/20 of children.
CRP concentration was statistically significantly higher in patients with bronchoobstruction in comparison with group of control children (Day 1 vs. control: P < 0.001; Day 3 vs. control: P < 0.001) (Table 1). Also, CRP was higher pretherapeutically than on the third day of drug administration in children with moderate bronchoobstruction (P = 0.002), but not in children with severe bronchoobstruction. At admission, CRP was higher in children with moderate bronchoobstruction than in those with severe bronchoobstruction (P = 0.020). The highest CRP value (44.0 mg/L) was recorded in non-asthmatic child (aged 3 years) with moderate bronchoconstrition.
Magnesium and calcium showed significant correlation in patients at admission (r = 0.545; P = 0.002) but not after therapy (r = 0.353; P = 0.052). Also, correlation was confirmed between magnesium and calcium concentration in subgroup of patients with moderate bronchoobstruction (r = 0.528; P = 0.021) as well as in patients with severe bronchoconstriction (r = 0.641; P = 0.043) (Figure 2). There was no correlation between magnesium, calcium, magnesium/calcium ratio and concentration of CRP. Also, selected biomarkers were related to neither leukocyte number nor number of lymphocytes and neutrophils (data not presented).
Rate of asthmatic was higher in group of children with severe bronchoconstriction.
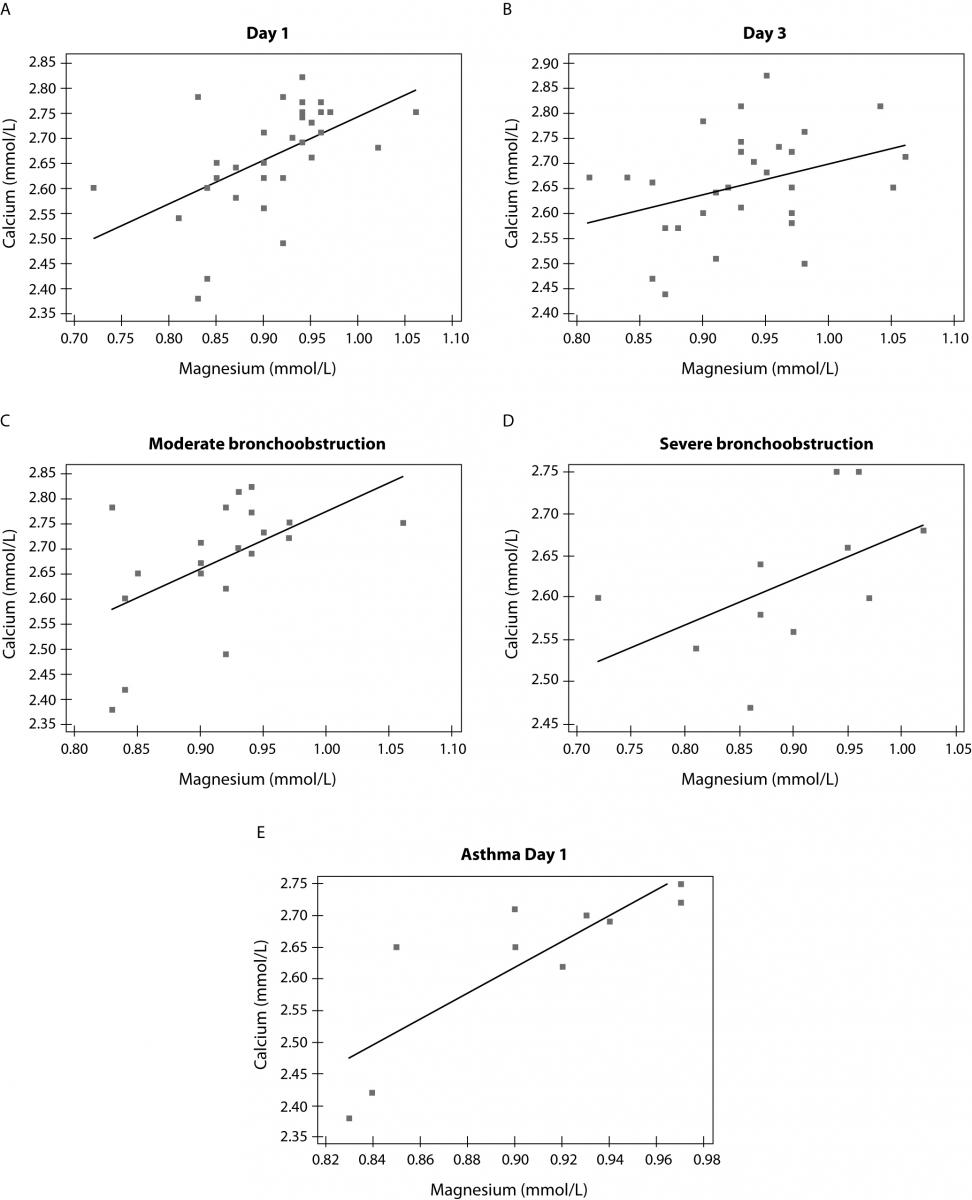
Figure 2. Correlation between calcium and magnesium in children at day 1 (A) (r = 0.545; P = 0.002), day 3 (B) (r = 0.353; P = 0.052), in subgroups of children with moderate, (C) (r = 0.528; P = 0.021) and severebronchoobstruction, (D) (r = 0.641; P = 0.043), and at day 1 in asthmatic children with moderate bronchoconstriction (E) (r = 0.826; P = 0.003).
Discussion
Study results have shown increased serum concentration of CRP during three days follow-up in children with bronchoconstriction cause by respiratory viral infection in comparison to control group of children. That increase before salbutamol administration (but not on the third day of therapy) was greater than in children with moderate bronchoconstriction. During follow-up period, magnesium concentration was higher in children with moderate (but not in severe) bronchoconstriction than in healthy children, with consequently higher magnesium to calcium ratio. We hypothesize that CRP values might be in correlation with degree of inflammation caused by viral infection. Furthermore, magnesium and magnesium to calcium ratio were possibly increased due to the relaxation of bronchial smooth muscle facilitated by magnesium.
Viral infections, especially those caused by adenoviruses and respiratory syncytial virus, could significantly increase serum CRP values (17,18).
In viral infections levels of CRP could be higher than 40 mg/L, and in some children even higher than 80 mg/L (19). In the present study, the highest CRP value occurred in non-asthmatic child with moderate bronchoconstrition.
Hypomagnesemia could lead to bronchial smooth muscle contraction or lack of bronchial smooth muscle relaxation (20). In the present study hypomagnesemia was not recorded in either of patients. Contrary, initial serum magnesium concentration was higher in children with moderate bronchoconstriction (but not with severe bronchoconstriction) than in healthy children. It could be presumed that these patients have moderate bronchoobstruction just because of a serum magnesium levels sufficient to redistribute intracellularly. Concentration of magnesium in various cell types is a better indicator of magnesium homeostasis orredistribution in patients with bronchoconstriction than serum magnesium concentration (11,21). Also, magnesium to calcium ratio was highest on third day of salbutamol administration. Tight calcium to magnesium correlation recorded in subgroups of children with moderate and severe bronchoobstruction and in asthmatic children with moderate bronchoobstruction indicated their close inter-reactivity. Our previous investigation has shown the magnesium to calcium ratio to be changed in exhaled breath condensate in children with asthma in comparison to healthy children (22).
Although suffering from some limitations (small number of children with severe bronchconstriction; determination of magnesium and calcium in serum, not in cells, determination of total serum magnesium and calcium, and not their ionized forms) our study demonstrated the concentration of magnesium, as a marker of smooth muscle relaxation, to undergo changes during salbutamol administration, especially in children with moderate bronchoobstruction caused by viral infection. CRP, as a biomarker of nonspecific immunity, decreased spontaneously by gradual disappearance of signs and symptoms of viral infection. Determination of serum magnesium and calcium concentrations and determination of their ratio are not sufficient enough to follow-up on effects of therapy. Additional studies of ionized forms of magnesium and calcium, their intracellular content, as well as concentration in exhaled breath condensate are needed.
Notes
Potential conflict of interest
None declared.
References
2. Tan WC. Viruses in asthma exacerbations. Curr Opin Pulm Med 2005;11:21-6.
3. Ren-Bin T, Shu-Jen C. Serum levels of eosinophil cationic protein and eosinophils in asthmatic children during a course of prednisolone therapy. Pediatr Pulmonol 2001;31: 121-5.
4. Takemura M, Matsumoto H, Niimi A, Ueda T, Matsuoka H, Yamaguchi M, et al. High sensitivity C-reactive protein in asthma. Eur Respir J 2006;27:908-12.
5. Spivey WH, Skobellof EM, Levin RM. Effect of magnesium chloride on rabbit bronchial smooth muscle. Ann Emerg Med 1990;19:1107-12.
6. Ciarallo L, Sauer A, Shannon MW. Intravenous magnesium therapy for moderate to severe pediatric asthma: results of a randomized, placebo-controlled trial. J Pediatr 1996;129:809-14.
7. Zaloga GP, Roberts PR. Calcium, phosphorus and magnesium disorders. Ayres SM, Greuvik NA, Holbrook PR, et al. eds. Textbook of critical care 4th ed. 2000, 905-928 WB Saunders, Philadelphia.
8. Cairns CB, Kraft M. Magnesium attenuates the neutrophils respiratory burst in adult asthmatic patients. Acad Emerg Med 1996;3:1093-7.
9. Hasebe N. Oxidative stress and magnesium. Clin Calcium 2005;15:194-202.
10. Emelyanov A, Fedoseev G, Barnes PJ. Reduced intracellular magnesium concentration in asthmatic patients. Eur Respir J 1999;13:38-40.
11. Mirčetić Novak R, Dodig S, Raos M, Petres B, Čepelak I. Magnesium concentration in plasma, leukocytes and urine of children with intermittent asthma. Clin Chim Acta 2001;312:197-203.
12. Raos M, Dodig S. [Praćenje koncentracije C-reaktivnog proteina u djece s astmom]. Paediatr Croat 2007;51:105-9. (in Croatian)
13. Galez D, Dodig S, Raos M, Nogalo B. C-reactive protein in children with asthma and allergic rhinitis. Biochem Med 2006;16:163-9.
14. Bacharier LB, Bonner A, Carlsen KH, Eigenmann PA, Frischer T, Götz M, et al. European Pediatric Asthma Group. Diagnostis and treatment of asthma in childhood: a PRACTALL consensus report. Allergy 2008;63:5-34.
15. Dupuy AM, Badiou S, Descomps B, Cristol JP. Immunoturbidimetric determination of C-reactive protein (CRP) and high sensitive CRP on heparin plasma. Comparison with serum determination. Clin Chem Lab Med 2003;41:948-9.
16. Marusteri M, Bacarea V. Comparing groups for statistical differences: how to choose the right statistical test? Biochem Med 2010;20:15-32.
17. Peltola V, Mertsola J, Ruuskanen O. Comparison of total white blood cell count and serum C-reactive protein levels in confirmed bacterial and viral infections. J Pediatr 2006;149:721-4.
18. Lukić-Grlić A, Baće A., Lokar-Kolbas R, Loffler-Badžek D, Draženović V, Božikov J., Mlinarić-Galinović G. Clinical and epidemiological aspects of respiratory syncytial virus lower respiratory tract infections. Eur J Epidem 1999;15:361-5.
19. Korppi M, Kröger L. C-reactive protein in viral and bacterial respiratory infection in children. Scand J Infect Dis 1993;25:207-13.
20. de Valk HW, Kok PT, Struyvenberg A, van Rijn HJ, Haalboom JR, Kreukniet J, Lammers JW. Extracellular and intracellular magnesium concentrations in asthmatic patients. Eur Respir J. 1993;6:1122-5.
21. Saris NEL, Mervaala E, Karppanen H, Khawaja J, Lewenstam A. Magnesium: an update on physiological, clinical and analytical aspects. Clin Chim Acta 2000;294:1-26.
22. Dodig S, Vlašić Ž, Čepelak I, Zrinski Topić R, Turkalj M, Nogalo B. Magnesium and calcium in exhaled breath condensate of children with asthma and gastroesophageal reflux disease. J Clin Lab Anal 2009;23:34-9.