Introduction
Laboratory testing is an integral part of the decision-making process, and results of laboratory testing often strongly influence medical diagnoses and therapies. There is a long history of quality requirements in laboratory medicine, which have mainly concerned the analytic phase of this process (1). Owing to the substantial advances in technology, laboratory automation and analytic quality, there is increasing evidence that further quality improvements should be targeted to extra-analytic phases of laboratory testing (2-6). Clinical laboratories routinely use commercial diagnostic products during the testing process. Diagnostic products can be divided into two major categories: in vitro diagnostic (IVD) devices, such as laboratory instruments, reagents, assays and blood collection tubes, and medical devices, such as specimen collection devices (needles and sets) (7). Necessary improvements and potential sources of nonconformities, either technical or concerning the quality management system, shall be identified and all laboratory process shall be validated (8). Some IVD devices (e.g. blood collection vacuum tubes) are not validated before the quality laboratory managers decide to start using or to change the brand. The aim of this study was to validate five kinds of serum vacuum tubes for routine clinical chemistry laboratory testing.
Materials and methods
Study design
A group of 100 adult ambulatory patients of both genders, volunteers for this study, from Dante Pazzanese Cardiology Insitute, São Paulo city, Brazil, were evaluated between 1st September and 1st October 2011. This study was submitted to the Internal Review Board and approved by the local Human Research Ethics Committee. All volunteers signed an informed consent.
Collection of diagnostic blood specimens
The collection of all diagnostic blood specimens was performed from 8.00 to 9.00 AM during one week by a single, expert phlebotomist, according to the recommendations of the Clinical Laboratory Standard Institute (CLSI) (9). All volunteers, after 12-hours fasting, were maintained seated for 15 minutes prior to phlebotomy in order to eliminate possible interferences of blood distribution due to the posture (10). After this time interval, a vein was located on forearm by a subcutaneous tissue transilluminator device (Venoscópio IV plus, Duan do Brasil, Sao Paulo, Brazil) to prevent interference from venous stasis (11-13), and 23.9 mL of blood was collected by venipuncture with a 20 G straight needle (Terumo Europe NV, Leuven, Belgium) directly into five serum vacuum tubes with clot activator and gel separator of different brands, as follows:
- Tube I: VACUETTE® 4.0 mL (lot C080818, Greiner Bio-one GmbH, Kremsmünster, Austria);
- Tube II: LABOR IMPORT® 6.0 mL (lot C38005-6, Guangzhou Improve Medical Instruments Co. Ltda, Zhejiang, China);
- Tube III: S-Monovette® 4.9 mL (lot 8092506, Sarstedt, Nümbrecht, Germany);
- Tube IV: SST® 4.0 mL (lot 8308434, Becton, Dickinson and Company Franklin Lakes, NJ, USA); and
- Tube V: SST II Advance® 5.0 mL (lot 8225174, Becton, Dickinson and Company Franklin Lakes, NJ, USA).
To eliminate any potential interference due to either the contact phase or the tissue factor, ~2 mL of blood were preliminarily collected in a discard tube without additive (Vacuette® lot A101004D, Greiner Bio-One GmbH, Kremsmünster, Austria). The exact composition of the gel separator and amount of clot activator inside the vacuum tubes was not communicated by manufactures, as patented. Blood collection was accurately standardized, including the use of needles and vacuum tubes of the same lot.
Processing of diagnostic blood specimens
All the sample tubes were left in upright position for 45 min at room temperature (20 ºC) to allow complete blood clotting before centrifugation (14).After centrifugation at 1500 x g for 10 min at room temperature (according to the instructions of the manufacturers), serum was separated, stored in aliquots and kept frozen at -70 ºC until measurement. All samples did not show any sign of haemolysis by visual inspection. No specimen was discarded due to unsatisfactory attempts, difficulty in locating venous access, missing veins, manifest haemolysis or lipaemia.
Laboratory testing
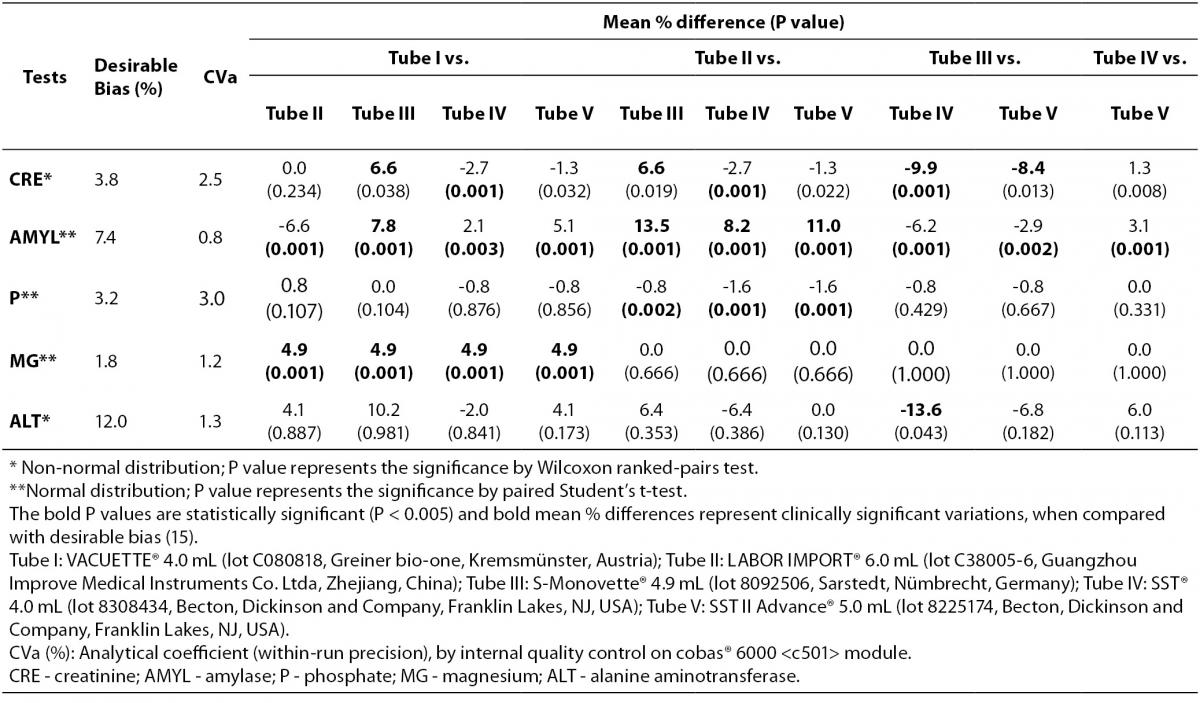
All serum aliquots were thawed at the same time. The routine clinical biochemistry tests were performed in duplicate immediately after thawing on the same instrument cobas® 6000 <c501> module (Roche Diagnostics GmbH, Penzberg, Germany), according to the manufacturer’s specifications and using proprietary reagents. The panel of tests included the following: glucose (GLU), total cholesterol (COL), high density lipoprotein-cholesterol (HDL), triglycerides (TG), total protein (TP), albumin (ALB), blood urea nitrogen (UREA), creatinine (CRE), uric acid (AU), alkaline phosphatase (ALP), amylase (AMYL), aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyltransferase (GGT), lactate dehydrogenase (LDH), creatine kinase (CK), total bilirubin (BT), direct bilirubin (BD), phosphate (P), calcium (CA), magnesium (MG), iron (FE), sodium (NA) and potassium (K). The instrument was calibrated against appropriate proprietary reference standard material and verified with the use of proprietary quality controls. Our evaluation of the within-run precision by internal quality control on cobas® 6000 <c501> module showed low coefficients of variation (Table 1).
Table 1. Variability in routine clinical biochemistry testing from five different brands of serum vacuum tubes with clot activator and gel separator.
Statistical analysis
The significance of the differences between samples was assessed by repeated measures using RM ANOVA and paired Student’s t-test after checking for normality (with D’Agostino-Pearson’s omnibus test). As non-normal distribution was found for GLU, CRE, AU, ALT, GGT, CK, BT, FE and NA results were assessed by Friedman test and Wilcoxon ranked-pairs test using licensed statistical software (GraphPad Prism® version 5.01, La Jolla, CA, USA). Based on screening five different vacuum tubes in parallel, a P value < 0.005 was considered statistically significant according to Bonferroni correction for multiple comparisons. Finally, the biases from Tube I, Tube II, Tube III, Tube IV and Tube V were compared with the current desirable quality specifications for bias (B), derived from biological variation according to the formula B < 0.25 (CVw2 + CVg2)1/2 where CVw and CVg are within- and between-subject CVs (15).
Results
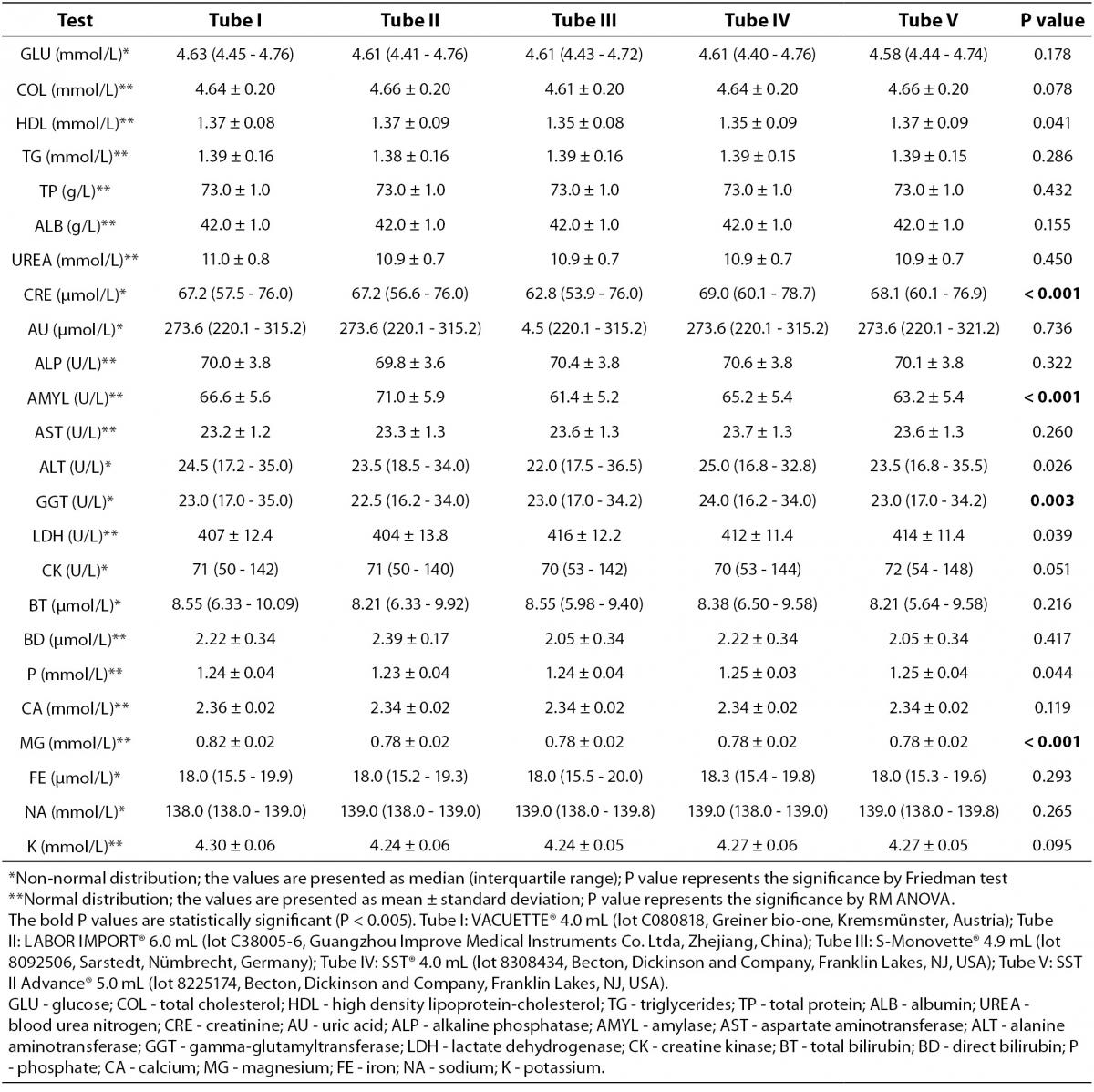
The main results of this study are shown in tables 1 and 2. Basically, significantly differences could be recorded for the following: CRE when comparing Tube I vs. Tube IV, Tube II vs. Tube IV and Tube III vs. Tube IV; AMYL when comparing Tube I vs. Tube II, Tube I vs. Tube III, Tube I vs. Tube IV, Tube I vs. Tube V, Tube II vs. Tube III, Tube II vs. Tube IV, Tube II vs. Tube V, Tube III vs. Tube IV, Tube III vs. Tube V and Tube IV vs. Tube V; P when comparing Tube II vs. Tube III, Tube II vs. Tube IV and Tube II vs. Tube V; MG when comparing Tube I vs. Tube II, Tube I vs. Tube III, Tube I vs. Tube IV and Tube I vs. Tube V. No significant differences (P > 0.005) were observed for: GLU, COL, HDL, TG, TP, ALB, UREA, AU, ALP, AST, ALT,GGT, LDH, CK, BT, BD, CA, FE, NA and K. Clinically significant variations as compared with the current desirable quality specifications (15) were found for: CRE when comparing Tube I vs. Tube III (without statistical significance, P = 0.038), Tube II vs. Tube III (without statistical significance, P = 0.019), and Tube III vs. Tube IV and Tube III vs. Tube V (without statistical significance, P = 0.013); AMYL when comparing Tube I vs. Tube III, Tube II vs. Tube III, Tube II vs. Tube IV, and Tube II vs. Tube V; ALT only when comparing Tube III vs. Tube IV (without statistical significance, P = 0.043); MG when comparing Tube I vs. Tube II, Tube I vs. Tube III, Tube I vs. Tube IV and Tube I vs. Tube V.
Table 2. Comprehensive results of routine clinical biochemistry testing from five different brands of serum vacuum tubes with clot activator and gel separator.
Discussion
The validation process is essential in accredited clinical laboratories (8,16). Recently article published by Stankovic et al. (7) showed that IVD companies that are concerned about total quality in laboratory diagnostics have dedicated resources to assure total system performance. These individuals collaborate with colleagues from instrument/assay/tube companies to address particular customer complaints and find root cause and ways to mitigate it (7). In opposite way Bowen et al. showed that components from blood collection tube e.g. surfactants, stopper, stopper lubricant, separator gel and clot activator interact with blood affecting laboratory tests (17). Our results showed that both Stankovic et al. and Bowen et al. are partially correct. Basically, our validation will permit the laboratory or hospital managers to select the brand’s vacuum tubes validated according him/her technical or economical reasons, in order to perform the following laboratory tests: GLU, COL, TG, TP, ALB, UREA, AU, ALP, AST, CK, BT, BD, CA, FE, NA and K. On the contrary special attention will be required in the following situations: a) if the laboratory performs CRE determination and the quality laboratory manager intends to change the serum Tube IV to Tube- I, II or III; b) if the laboratory performs AMYL determination and the quality laboratory manager intends to change any of the tested brands of serum vacuum tubes; c) if the laboratory performs P determination and the quality laboratory manager intends to change the serum Tube II to Tube- III, IV or V; and d) if the laboratory already performs MG determination and the quality laboratory manager intends to change the serum Tube I to Tube- II, III, IV or V (Table 1). When looking at the above CRE, AMYL, P and MG results (Table 2) one might regard them as clinically irrelevant, though such a conclusion is far from correct in respect of the current quality specifications for bias, derived from biological variation (15). Obviously the quality specifications derived from biological variation (15) are considered both very important and useful in the daily practice by the quality managers of the medical laboratories (18-21). Even in this case caring physicians unaware of the real patient situation might abstain from appropriate treatments as a consequence of change in serum vacuum tubes brands. The National Kidney Foundation published the Kidney Disease Outcomes Quality Initiative (K/DOQI) Clinical Practice Guidelines for chronic kidney disease years ago (22). These guidelines recommend the use of estimated glomerular filtration rate (eGFR) equations to estimate a patient’s renal function. At the present time several articles have been published about, evidencing the impact of these guidelines in clinical practices daily (23-25). When considering that accurate serum creatinine determination is necessary for deriving the above eGRF equations, changes of serum vacuum tubes principally from Tube III to Tube IV (with clinically significant difference) can induce diagnostic errors regarding patient real conditions. Acute pancreatitis is an acute inflammatory condition of the pancreas, which might extend to local and distant extra pancreatic tissues. Diagnosis of acute pancreatitis is substantially based on a combination of clinical signs and symptoms, imaging techniques and laboratory investigations (26). A host of serum enzymes such as amylase, lipase, trypsinogen, elastase, phospholipase A2, ribonuclease, etc are available to diagnose acute pancreatitis and/or to assess the severity, but elevated amylase levels continue to be the “gold standard” among the serum markers (27). Our results show that the changes in serum vacuum tubes brands principally from Tube II to Tube- III or V can manifest clinically significant impact in medical decisions based on laboratory diagnosis. As for phosphate, the vacuum tubes change from Tube II to Tube- III, IV or V can significantly influence phosphates levels; in this respect we must consider that: a) Ferrari et al. (28) showed that serum phosphate is an important determinant for correcting serum calcium in end-stage kidney disease, b) this retains even if the target concentrations for phosphorus and calcium × phosphorus product are sometimes close to the normal range even in patients with end-stage kidney disease (28), c) a relationship between serum phosphate and cardiovascular risk factors was demonstrated by Lippi et al. (29). The fourth most abundant cation in the human body, magnesium is, like potassium, predominantly intracellular. It is critically involved in energy metabolism, enzyme functions and participates in the regulation of PTH synthesis, release, and action (30). Low magnesium levels have been associated with impairment of myocardial contractility, intradialytic hemodynamic instability, and hypotension. In addition, low MG has been also linked to carotid intima-media thickness, a marker of atherosclerotic vascular disease and a predictor of vascular events (31). Magnesium sulphate is also acknowledged as the preferred anticonvulsant for eclamptic women, since it reduces the risk ratio of recurrence of seizures, probably reduces the risk of maternal death, and improves outcome for the children (32). Even for MG, inappropriately high values due to changes in vacuum tubes brands (from Tube I to Tube- II, III, IV or V) might induce diagnostic errors regarding patient real conditions. The 5 kinds of serum vacuum tubes evaluated were validated for 83.3% of the tests but obviously 16.7% of the routine clinical chemistry testing needs more attention when the lab intends to change the serum vacuum tubes brands. In the same way Lima-Oliveira et al. have shown recently that different manufacturing source of syringes is a new source of extra analytical variability in blood gas analyses (33). Similarly, as the concentration of clot activator additives as well as gel composition in the serum tubes are patented, the implicit “industrial secret” does not aid to clarify the causes of the bias observed in our study though it is strongly hypothesized, basing on Bowen et al. (17) arguments. Future investigations should be planned to better understand the nature of this intriguing observations; in the meantime we suggest that every laboratory management should both standardize the procedures and frequently evaluate the quality of IVD devices.
Notes
Potential conflict of interest
None declared.
References
1. Lippi G, Simundic AM. Total quality in laboratory diagnostics. It’s time to think outside the box. Biochem Med 2010;20:5-8.
2. Simundic AM, Bilic-Zulle L, Nikolac N, Supak-Smolcic V, Honovic L, Avram S, et al. The quality of the extra-analytical phase of laboratory practice in some developing European countries and Mexico - a multicentric study. Clin Chem Lab Med 2011;49:215-28.
3. Bilic-Zulle L, Simundic AM, Smolcic VS, Nikolac N, Honovic L. Self reported routines and procedures for the extra-analytical phase of laboratory practice in Croatia -cross-sectional survey study. Biochem Med 2010;20:64-74.
4. Lippi G, Salvagno GL, Montagnana M, Franchini M, Guidi GC. Phlebotomy issues and quality improvement in results of laboratory testing. Clin Lab 2006;52:217-30.
5. Lippi G, Lima-Oliveira G, Nazer SC, Moreira ML, Souza RF, Salvagno GL, et al. Suitability of a transport box for blood sample shipment over a long period. Clin Biochem 2011;44:1028-9.
6. Lima-Oliveira G, Guidi GC, Salvagno GL, Montagnana M, Rego FGM, Lippi G, et al. Is phlebotomy part of the dark side in the clinical laboratory struggle for quality? Laboratory Medicine Manuscript ID 11-08-LM-U-SCI-0152.R1, In press.
7. Stankovic AK, Silvestri J, Mails M, Najork C. Total quality in laboratory diagnostics: the role of commercial companies. Biochemia Medica 2010;20:207-14.
8. ISO. Medical laboratories — Particular requirements for quality and competence ISO 15189. 2 ed 2007.
9. CLSI. Procedures for the collection of diagnostic blood specimens by venipuncture. NCCLS H3-A6. 6 ed 2007.
10. Guder WG, Narayanan S, Wisser H, Zawta B. Diagnostic samples: from the patient to the laboratory: the impact of preanalytical variables on the quality of laboratory results. 4 ed: Wiley-Blackwell; 2009.
11. Lima-Oliveira G, Lippi G, Salvagno GL, Montagnana M, Scartezini M, Guidi GC, et al. Transillumination: a new tool to eliminate the impact of venous stasis during the procedure for the collection of diagnostic blood specimens for routine haematological testing. Int J Lab Hematol 2011;33:457-62.
12. Lima-Oliveira G, Salvagno GL, Lippi G, Montagnana M, Scartezini M, Picheth G, et al. Elimination of the venous stasis error for routine coagulation testing by transillumination. Clin Chim Acta 2011;412:1482-4.
13. Lima-Oliveira G, Lippi G, Salvagno GL, Montagnana M, Mangueira C, Sumita N, et al. New ways to deal with known preanalytical issues: use of transilluminator instead of tourniquet for easing vein access and eliminating stasis on clinical biochemistry. Biochem Med 2011;21:152-9.
14. CLSI. Procedures for the handling and processing of blood specimens for common laboratory tests. NCCLS H18-A4. 4 ed 2010.
15. Ricos C, Alvarez V, Cava F, Garcia-Lario JV, Hernandez A, Jimenez CV, et al. Current databases on biological variation: pros, cons and progress. Scand J Clin Lab Invest 1999;59:491-500.
16. Yanikkaya-Demirel G. ISO 15189 accreditation: Requirements for quality and competence of medical laboratories, experience of a laboratory II. Clin Biochem 2009;42:279-83.
17. Bowen RA, Hortin GL, Csako G, Otanez OH, Remaley AT. Impact of blood collection devices on clinical chemistry assays. Clin Biochem 2010;43:4-25.
18. Ricos C, Cava F, Garcia-Lario JV, Hernandez A, Iglesias N, Jimenez CV, et al. The reference change value: a proposal to interpret laboratory reports in serial testing based on biological variation. Scand J Clin Lab Invest 2004;64:175-84.
20. Cembrowski GS, Tran DV, Higgins TN. The use of serial patient blood gas, electrolyte and glucose results to derive biologic variation: a new tool to assess the acceptability of intensive care unit testing. Clin Chem Lab Med 2010;48:1447-54.
21. Plebani M, Lippi G. Biological variation and reference change values: an essential piece of the puzzle of laboratory testing. Clin Chem Lab Med 2012;50:189-90.
22. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002;39:S1-266.
23. Jain AK, Cuerden MS, McLeod I, Hemmelgarn B, Akbari A, Tonelli M, et al. Reporting of the estimated glomerular filtration rate was associated with increased use of angiotensin-converting enzyme inhibitors and angiotensin-II receptor blockers in CKD. Kidney Int 2012;DOI 10.1038/ki.2012.18.
24. Kagoma YK, Garg AX, Li L, Jain AK. Reporting of the estimated glomerular filtration rate decreased creatinine clearance testing. Kidney Int 2012;DOI 10.1038/ki.2011.483.
25. Geara AS, Azzi N, Ghimire P, Abdallah M, Siddiqui A, Bassil C, et al. The impact of reporting estimated glomerular filtration rate. Ren Fail 2011;33:486-8.
26. Lippi G, Valentino M, Cervellin G. Laboratory diagnosis of acute pancreatitis: in search of the Holy Grail. Crit Rev Clin Lab Sci 2012;49:18-31.
27. Yadav D, Agarwal N, Pitchumoni CS. A critical evaluation of laboratory tests in acute pancreatitis. Am J Gastroenterol 2002;97:1309-18.
28. Ferrari P, Singer R, Agarwal A, Hurn A, Townsend MA, Chubb P. Serum phosphate is an important determinant of corrected serum calcium in end-stage kidney disease. Nephrology (Carlton) 2009;14:383-8.
29. Lippi G, Montagnana M, Salvagno GL, Targher G, Guidi GC. Relationship between serum phosphate and cardiovascular risk factors in a large cohort of adult outpatients. Diabetes Res Clin Pract 2009;84:e3-5.
30. Kasama RK. Trace minerals in patients with end-stage renal disease. Semin Dial 2010;23:561-70.
31. Navarro-Gonzalez JF, Mora-Fernandez C, Garcia-Perez J. Clinical implications of disordered magnesium homeostasis in chronic renal failure and dialysis. Semin Dial 2009;22:37-44.
32. Duley L H-SD, Chou D. Magnesium sulphate versus phenytoin for eclampsia. Cochrane Database Syst Rev. 2010;(10):CD000128.
33. Lima-Oliveira G, Lippi G, Salvagno GL, Montagnana M, Picheth G, Guidi GC. Different manufacturers of syringes: A new source of variability in blood gas, acid-base balance and related laboratory test? Clin Biochem 2012;DOI 10.1016/j.clinbiochem.2012.03.007.