References
1. The World Health Organization MONICA Project (monitoring trends and determinants in cardiovascular disease): a major international collaboration. WHO MONICA Project Principal Investigators. J Clin Epidemiol 1988;41:105-14.
2. Seidman C, Kirkham F, Pavlakis S. Pediatric stroke: current developments. Curr Opin Pediatr 2007;19:657-62.
3. Dlamini N, Kirkham FJ. Stroke and cerebrovascular disorders. Curr Opin Pediatr 2009;21:751-61.
4. Raju TN, Nelson KB, Ferriero D, Lynch JK, NICHD-NINDS Perinatal stroke workshop participants. Ischemic perinatal stroke: summary of a workshop sponsored by the National Institute of Child Health and Human Development and the National Institute of Neurological Disorders and Stroke. Pediatrics 2007;120:609-16.
5. Lynch JK, Hirtz DG, deVeber G, Nelson KB. Report of the National Institute of Neurological Disorders and Stroke workshop on perinatal and childhood stroke. Pediatrics 2002;109:116-23.
6. Agrawal N, Johnston SC, Wu YW, Sidney S, Fullerton HJ. Imaging data reveal a higher pediatric stroke incidence than prior US estimates. Stroke 2009;40:3415-21.
7. deVeber G, Andrew M, Adams C, Bjornson B, Booth F, Buckley DJ, et al. Cerebral sinovenous thrombosis in children. N Engl J Med 2001;345:417-23.
8. Laugesaar R, Kolk A, Uustalu Ü, Ilves P, Tomberg T, Talvik I, et al. Epidemiology of childhood stroke in Estonia. Pediatr Neurol 2010;42:93-100.
9. Kenet G, Waldman D, Lubetsky A, Kornbrut N, Khalil A, Koren A, et al. Paediatric cerebral sinus vein thrombosis: a multi-center, case-controlled study. Thromb Haemost 2004;92:713-8.
10. Laugesaar R, Kolk A, Tomberg T, Metsvaht T, Lintrop M, Varendi H, et al. Acutely and retrospectively diagnosed perinatal stroke: A population based study. Stroke 2007;38:2234-40.
11. Lanni G, Catalucci A, Conti L, Di Sibio L, Paonessa A, Galluci M. Pediatric stroke: clinical findings and radiological approach. Stroke Res Treat 2011; doi:10.4061/2011/172168.
12. Dlamini N, Billinghurst L, Kirkham FJ. Cerebral venous (sinovenous) thrombosis in children. Neurosurg Clin N Am 2010;21:511-27.
13. Lanthier S, Carmant L, David M, Labrisseau A, deVeber G. Stroke in children: the coexistence of multiple risk factors predicts poor outcome. Neurology 2000;54:371-8.
14. Sträter R, Becker S, von Eckardstein A, Heinecke A, Gutsche S, Junker R, et al. Prospective assessment of risk factors for reccurent stroke during childhood - a 5-year follow-up study. Lancet 2002;360:1540-5.
15. Kirkham FJ, Prengler M, Hewes KM, Ganesan V. Risk factors for arterial ischemic stroke in children. J Child Neurol 2000;299-307.
16. Ganesan V, Prengler M, McShane MA, Wade AM, Kirkham FJ. Investigation of risk factors in children with arterial ischemic stroke. Ann Neurol 2003;53:167-73.
17. Moll S. Thrombophilias - practical implications and testing caveats. J Thromb Thrombolysis 2006;21:7-15.
18. de Moerloose P, Boehlen F. Inherited thrombophilia in arterial disease: a selective revue. Semin Hematol 2007;44:106-13.
19. Colman RW, Hirsch J, Marder VJ, Clowes AW, George JN, eds. Hemostasis and thrombosis: basic principles and clinical practice. 4th ed. Philadelphia, PA: Lippincott Williams and Wilkins, 2001.
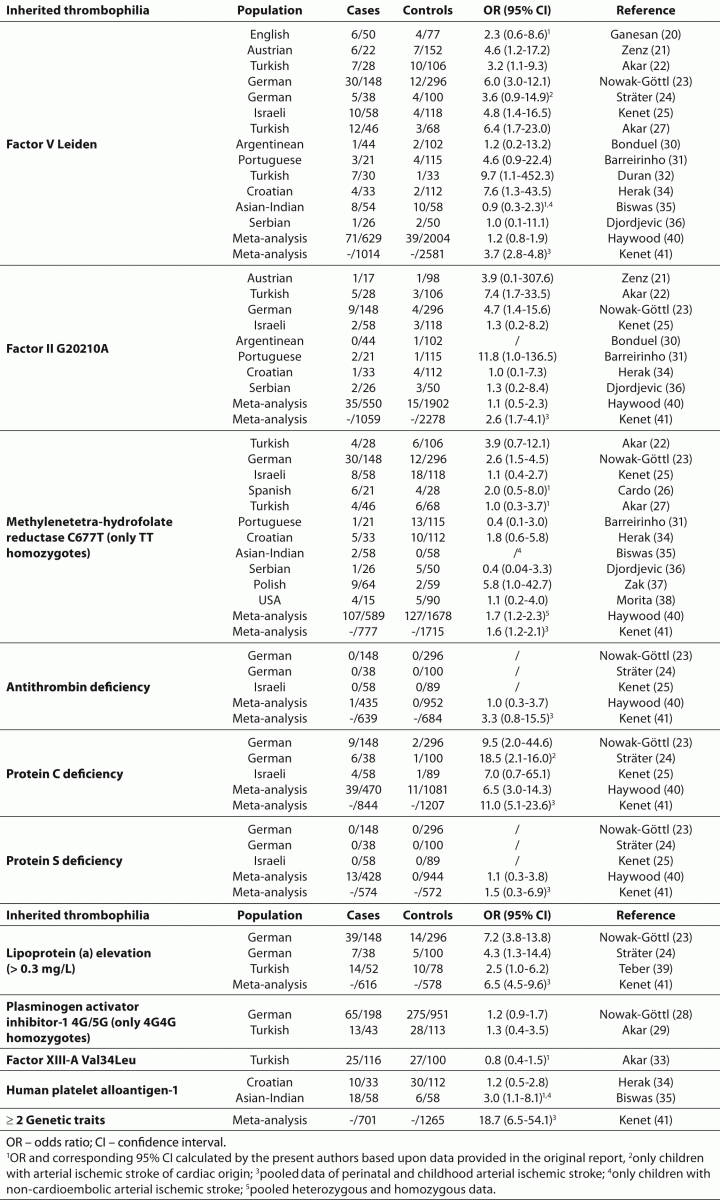
20. Ganesan V, McShane MA, Liesnet R, Cookson J, Hann I, Kirkham FJ. Inherited prothrombotic states in ischaemic stroke in childhood. J Neurol Neurosurg Psychiatry 1998;65:508-11.
21. Zenz W, Bodo Z, Plotho J, Streif W, Male C, Bernert G, et al. Factor V Leiden and prothrombin gene G 20210 A variant in children with ischemic stroke. Thromb Haemost 1998;80:763-6.
22. Akar N, Akar E, Deda G, Sipahi T, Orsal A. Factor V1691 G-A, prothrombin 20210 G-A, and methylenetetrahydrofolate reductase 677 C-T variants in Turkish children with cerebral infarct. J Child Neurol 1999;14:749-51.
23. Nowak-Göttl U, Sträter R, Heinecke A, Junker R, Koch HG, Schuierer G, et al. Lipoprotein (a) and genetic polymorphisms of clotting factor V, prothrombin, and methylenetetrahydrofolate reductase are risk factors of spontaneous ischemic stroke in childhood. Blood 1999;94:3678-82.
24. Sträter R, Vielhaber H, Kassenböhmer R, von Kries R, Göbel U, Nowak-Göttl U. Genetic risk factors of thrombophilia in ischaemicnchildhood stroke of cardiac origin: a prospective ESPED survey. Eur J Pediatr 1999;158(suppl 3):S122-5.
25. Kenet G, Sadetzki S, Murad H, Martinowitz U, Rosenberg N, Gitel S, et al. Factor V Leiden and antiphospholipid antibodies are significant risk factors for ischemic stroke in children. Stroke 2000;31:1283-8.
26. Cardo E, Monrós E, Colomé C, Artuch R, Campistol J, Pineda M, et al. Children with stroke: polymorphism of the MTHFR gene, mild hyperhomocysteinemia, and vitamin status. J Child Neurol 2000;15:295-8.
27. Akar N, Akar E, Ozel D, Deda G, Sipahi T. Common mutations at the homocysteine metabolism pathway and pediatric stroke. Thromb Res 2001;102:115-20.
28. Nowak-Göttl U, Sträter R, Kosch A, von Eckardstein A, Schobbes R, Luigs P, et al. The plasminogen activator inhibitor (PAI)-1 promoter 4G/4G genotype is not associated with ischemic stroke in a population of German children. Childhood Stroke Study Group. Eur J Haematol 2001;66:57-62.
29. Akar N, Akar E, Yilmaz E, Deda G. Plasminogen activator Inhibitor-1 4G/5G polymorphism in turkish children with cerebral infarct and effect on factor V 1691A mutation. J Child Neurol 2001;16:294-5.
30. Bonduel M, Sciuccati G, Hepner M, Pieroni G, Torres AF, Mardaraz C, et al. Factor V Leiden and prothrombin gene G20210A mutation in children with cerebral thromboembolism. Am J Hematol 2003;73:81-6.
31. Barreirinho S, Ferro A, Santos M, Costa E, Pinto-Basto J, Sousa A, et al. Inherited and acquired risk factors and their combined effects in pediatric stroke. Pediatr Neurol 2003;28:134-8.
32. Duran R, Biner B, Demir M, Celtik C, Karasalihoglu S. Factor V Leiden mutation and other thrombophilia markers in childhood ischemic stroke. Clin Appl Thromb Haemost 2005;11:83-8.
33. Akar N, Dönmez B, Deda G. FXIII gene Val34Leu polymorphism in Turkish children with cerebral infarct. J Child Neurol2007;22;222-4.
34. Herak DC, Antolic MR, Krleza JL, Pavic M, Dodig S, Duranovic V, et al. Inherited prothrombotic risk factors in children with stroke, transient ischemic attack and migraine. Pediatrics 2009;123:e653-60.
35. Biswas A, Tiwari AK, Ranjan R, Meena A, Akhter MS, Yadav BK, et al. Prothrombotic polymorphisms, mutations, and their association with pediatric non-cardioembolic stroke in Asian-Indian patients. Ann Hematol 2009;88:473-8.
36. Djordjevic V, Stankovic M, Brankovic-Sreckovic V, Rakicevic Lj, Radojkovic D. Genetic risk factors for arterial ischemic stroke in children: a possible MTHFR and eNOS gene-gene interplay? J Child Neurol 2009;24:823-7.
37. Zak I, Sarecka-Hujar B, Kopyta I, Emich-Widera E, Marszal E, Wendorff J, et al. The T allele of the 677C>T polymorphism of methylenetetrahydrofolate reductase gene is associated with an increased risk of ischemic stroke in Polish children. J Child Neurol 2009;24:1262-7.
38. Morita DC, Donaldson A, Butterfield RJ, Benedict SL, Bale JF. Methylenetetrahydrofolate reductase gene polymorphism and childhood stroke. Pediatr Neurol 2009;41:247-9.
39. Teber S, Deda G, Akar N, Soylu K. Lipoprotein (a) levels in childhood arterial ischemic stroke. Clin Appl Thromb Hemost 2010;16:214-7.
40. Haywood S, Liesner R, Pindora S, Ganesan V. Thrombophilia and first arterial ischaemic stroke: a systematic review. Arch Dis Child 2005;90:402-5.
41. Kenet G, Lutkhoff LK, Albisetti M, Bernard T, Bonduel M, Brandao L, et al. Impact of thrombophilia on arterial ischemic stroke or cerebral sinovenous thrombosis in children: a systematic review & meta analysis of observational studies. Circulation 2010;121.1838-47.
42. Caplice NM, Panetta C, Peterson TE, Kleppe LS, Mueske CS, Kostner GM, et al. Lipoprotein (a) binds and inactivates tissue factor pathway inhibitor: a novel link between lipoproteins and thrombosis. Blood 2001;98:2980-7.
43. Cnossen MH, van Ommen CH, Appel IM. Etiology and treatment of perinatal stroke; a role for prothrombotic coagulation factors? Semin Fetal Neonatal Med 2009;14:311-7.
44. Günther G, Junker R, Sträter R, Schobess R, Kurnik K, Heller C, et al. Symptomatic ischemic stroke in full-term neonates: role of acquired and genetic prothrombic risk factors. Stroke2000;31:2437-41.
45. Kurnik K, Kosch A, Sträter, R, Schobess R, Heller C, Nowak-Gättl U, for the Childhood Stroke Study Group. Recurrent thromboembolism in infants and children suffering from symptomatic neonatal arterial stroke – a prospective follow-up study. Stroke 2003;34:2887-93.
46. Debus OM, Kosch A, Sträter R, Rossi R, Nowak-Göttl U. The factor V G1691A mutation is a risk for porencephaly: a case-control study. Ann Neurol 2004;56:287-90.
47. Miller SP, Wu YW, Lee J, Lammer EJ, Iovannisci DM, Glidden DV, et al. Candidate gene polymorphisms do not differ between newborns with stroke and normal controls. Stroke 2006;37:2678-83.
48. Simchen MJ, Goldstein G, Lubetsky A, Strauss T, Schiff E, Kenet G. Factor V Leiden and antiphospholipid antibodies in either mothers or infants increase the risk for perinatal arterial ischemic stroke. Stroke 2009;40:65-70.
49. Curry CJ, Bhullar S, Holmes J, Delozier CD, Roeder ER, Hutchison HT. Risk factors for perinatal arterial stroke: a study of 60 mother-child pairs. Pediatr Neurol 2007;37:99-107.
50. Laugesaar R, Kahre T, Kolk A, Uustalu U, Kool P, Talvik T. Factor V Leiden and prothrombin 20210G>A [corrected] mutation and paediatric ischaemic stroke: a case-control study and two meta-analyses. Acta Pediatr 2010;99:1168-74.
51. Renaud C, Tardy-Poncet B, Presles E, Chabrier S; AVCnn group. Low prevalence of coagulation F2 and F5 polymorphisms in mothers and children in a large cohort of patients with neonatal arterial ischemic stroke. Br J Haematol 2010;150:709-12.
52. Hagstrom JN, Walter J, Bluebond-Langner R, Amatniek JC, Manno CS, High KA. Prevalence of the factor V Leiden mutation in children and neonates with thromboembolic disease. J Pediatr 1998;133:777-81.
53. Heller C, Heinecke A, Junker R, Knöfler R, Kosch A, Kurnik K, et al. Cerebral venous thrombosis in children-a multifactorial etiology.Circulation 2003;108:1362-7.
54. Simundic AM. Methodological issues of genetic association studies. Clin Chem Lab Med 2010;48:S115-8.
55. SébireG, Fullerton H, Riou E, deVeber G. Toward the definition of cerebral arteriopathies of childhood. Curr Opin Pediatr 2004;16:617-22.
56. Sofronas M, Ichord RN, Fullerton HJ, Lynch JK, Massicotte MP, Willan AR, et al. Pediatric stroke initiatives and preliminary studies. What is known and what is needed? Pediatr Neurol 2006;34:439-45.
57. Amlie-Lefond C, deVeber G, Chan AK, Benedict S, Bernard T, Carpenter J, et al. International Pediatric Stroke Study. Use of alteplase in childhood arterial ischemic stroke: a multicentre, observational cohort study. Lancet Neurol 2009;8:530-6.
58. Amlie-Lefond C, Bernard TJ, Sébire G, Friedman NR, Heyer GL, Lerner NB, et al. International Pediatric Stroke Study group. Predictors of cerebral arteriopathy in children with arterial ischemic stroke: results of the International Pediatric Stroke Study. Circulation 2009;119:1417-23.
59. Goldenberg NA, Bernard TJ, Fullerton HJ, Gordon A, deVeber G; International Pediatric Stroke Study group. Antithrombotic treatments, outcomes, and prognostic factors in acute childhood-onset arterial ischemic stroke: a multicentre, observational, cohort study. Lancet Neurol 2009;8:1120-7.
60. Golomb MR, Fullerton HJ, Nowak-Göttl U, deVeber G; International Pediatric Stroke Study group. Male predominance in childhood ischemic stroke: findings from the International Pediatric Stroke Study. Stroke 2009;40:52-7.
61. Jordan LC, Rafay MF, Smith SE, Askalan R, Zamel KM, deVeber G, et al. International Pediatric Stroke Study group. Antithrombotic treatment in neonatal cerebral sinovenous thrombosis: results of the the International Pediatric Stroke Study. J Pediatr 2010;156:704-10.
62. Kirton A, Amstrong-Wells J, Chang T, deVeber G, Rivkin MJ, Hernandez M, et al. The International Pediatric Stroke Study investigators. Symptomatic neonatal arterial ischemic stroke. The International Pediatric Stroke Study. Pediatrics 2011:128:e1402-10.
63. Mackay TM, Wiznitzer M, Benedict SL, Lee KJ, deVeber GA, Ganesan V. Arterial ischemic stroke risk factors: the International Pediatric Stroke Study group. Ann Neurol 2011;69:130-40.
64. Bernard TJ, Manco-Johnson MJ, Lo W, Mackay MT, Ganesan V, deVeber G, et al. Towards a consensus-based classification of childhood arterial ischemic stroke. Stroke 2012;43:371-7.
65. Barnes C, deVeber G. Prothrombotic abnormalities in childhood ischemic stroke. Thromb Res 2006;118:67-74.