Introduction
The presence of thyroglobulin autoantibodies (TgAb) in serum samples of patients with differentiated thyroid carcinoma (DTC) is a serious technical problem affecting the use of thyroglobulin (Tg) as a tumour marker, because TgAb interference may result in an underestimation of serum Tg concentrations. The prevalence of TgAb in patients with DTC is much higher (20-30%) than in the general population (1-5). This high prevalence, together with studies showing that even low levels of TgAb can cause falsely low Tg values may explain why all specimens need sensitive TgAb screening. However, current TgAb assays are qualitatively and quantitatively variable and TgAb concentrations obtained with different methods may show 100-fold differences in the same specimen (5,6). The manufacturers of current TgAb assays claim that the secondary standards included in their kits are calibrated against IRP-65/93 reference preparation as primary standard. The assay specific use of different secondary standards is one reason why different methods may provide different TgAb concentrations. These differences reflect not only suboptimal sensitivity and specificity but also different interactions between the patient-specific isoforms and the assay reagents (standards, tracer, antibody). These important variables explain why high TgAb concentrations do not always interfere, while in other cases low TgAb levels result in significant interference with the Tg assay (3,5,6).
Because there is no reliable TgAb reference method, it is necessary to use an independent parameter for seeking the presence of interfering TgAb. The Tg recovery test, which is based on the addition of a known quantity of Tg, proved to detect less reliably TgAb in some studies, and some, although not all studies discourage its use for the follow-up of patients with DTC (3-8). It has been also shown that radioimmunoassay (RIA) is less prone to the interfering effect of TgAb than other methods such as immunometric assays (IMA). In general, immunometric assays give lower serum Tg values in the presence of TgAb as compared to those obtained with RIA (3-6). In some studies the interference of TgAb with Tg measurement was estimated on the basis of discordant results of Tg measurements obtained with IMA and RIA (3-6).
Although recent studies confirmed that low TgAb concentrations may have an impact on the validity of Tg measurements (9,10).
Materials and Methods
Subjects
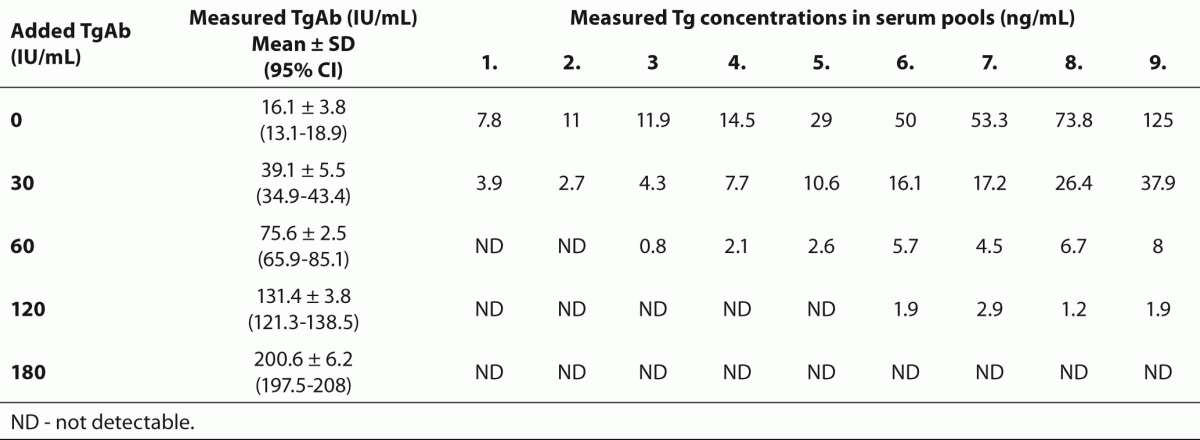
For our in vitro experiments we collected during a period of one year 9 serum pools with TgAb concentrations below the functional sensitivity of the TgAb assay.These serum samples were sent to the laboratory for TgAb measurement, and leftover samples were used for the experiments. All blood samples were taken under fasting condition between 08.00 and 10.00 AM. in 2 mL native vacoutainer tubes, then serum samples were aliquoted, stored at -80 oC and processed monthly. The Tg concentrations in these serum pools ranged from 7.8 to 125 ng/mL (Table 1).
Table 1. Measured Tg and TgAb concentrations in aliquots of the 9 serum pools after the addition of 0, 30, 60, 120 and 180 IU/mL TgAb.
Serum samples (N = 134) were also collected from 27 DTC patients after thyroidectomy and radioiodine ablation (22 women and 5 men; median age, 47 years; lower and upper age quartiles, 42 and 58 years, respectively). Median follow-up time was 2.5 years (lower and upper follow-up time quartiles, 0.5 and 8.0, respectively). The number of serum samples obtained from the same patient during follow-up was between 2 and 6. None of the DTC patients had previously diagnosed Graves’ disease or Hashimoto thyroiditis.All but 28 samples were obtained from patients receiving thyroxin (L-T4) treatment. Two of the 27 patients developed metastatic DTC during the follow-up period. The study was performed in the Central Laboratory, Markusovszky Teaching Hospital of County Vas, Szombathely, and was approved by the Research Ethics Committee of Markusovszky Hospital.
Methods
Increasing concentrations of TgAb (ECLIA kit calibrator containing 3250 IU/l polyclonal TgAb produced in sheep against human Tg and solved in human serum matrix; Roche GmbH, Germany, Mannheim) were added to aliquots of the 9 TgAb-free serum pools with varying concentrations of Tg to reach TgAb concentrations within or near to the reference range (Table 1). Samples were then incubated for one hour at room temperature and assayed for Tg and TgAb. Tg was undetectable in the TgAb solution.
Serum Tg and TSH concentrations were measured using electrochemiluminometric assays (ECLMA, Roche) and TgAb by an electrochemiluminescence immunoassay (ECLIA, Roche). Each method was carried out using an Elecsys 2010 automated immunochemical analyser (Roche).
The cutoff value for increased TgAb provided by the manufacturer was 115 IU/mL. The within-run coefficients of variations (CV) were 8.6%, 2.1% and 1.8% using samples containing 0.034, 0.91 and 3.96 IU/mL TgAb, respectively. The inter-assay CVs measured in serum samples with target TgAb levels of 115 ± 8.3 and 62.8 ± 5.4 IU/mL were 7.2 % and 8.7%, respectively (N = 21). The analytical sensitivity provided by the manufacturer was 10 IU/mL. We determined the functional sensitivity of the method in 8 serum pools with TgAb concentrations ranging from 10 to 110 IU/mL. The CV of > 19% was reached at 24 IU/mL and, therefore, values below this limit were considered as undetectable TgAb concentrations.
The intra-assay CVs for Tg measurements were 1.8% and 1.4% in serum samples containing 4.1 and 26.9 ng/mL Tg, respectively. The inter-assay CVs were 1.8% and 3.6% for samples containing 3 and 4.2 ng/mL Tg, respectively (N = 21). The analytical sensitivity was 0.1 ng/mL. We determined the functional sensitivity of the method in 7 serum pools with Tg concentrations ranging from 0.46 to 4.6 ng/mL. The CV of > 19% was reached at 0.46 ng/mL Tg concentration and, therefore, values below this limit were considered as undetectable Tg concentrations.
The reference range for the TSH assay was between 0.27 and 4.2 mU/L, and the functional sensitivity provided by the manufacturer was 0.014 mU/L.
Statistical analysis
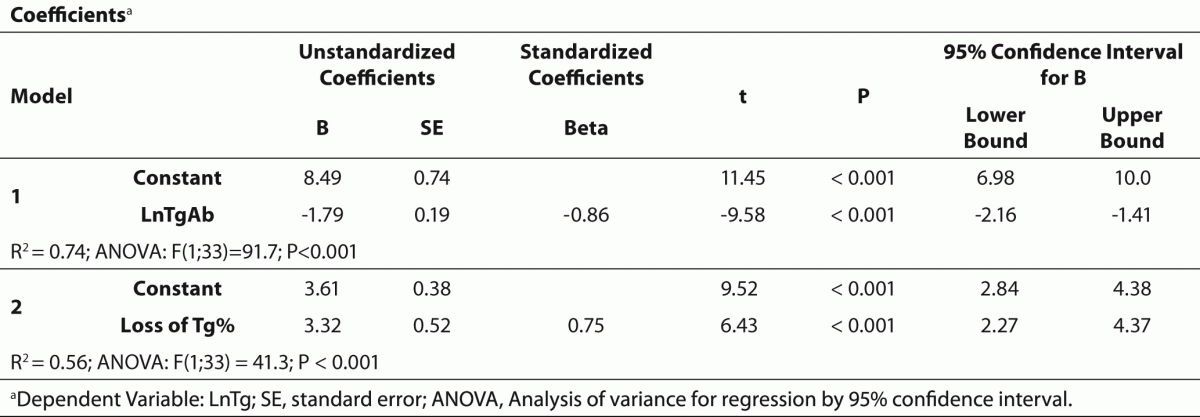
The values are presented as median and lower and upper quartiles (25% and 75% percentiles) except for in vitro experiments where the results are given as means ± SD. TgAb (as independent variable) and Tg concentrations obtained from in vitro experiments were logarithmically (Ln) transformed then their relationship was analyzed by linear regression. Changes in Tg concentrations expressed as percent decrease from baseline after the addition of increasing concentrations of TgAb were also analyzed. The association between loss of Tg% and TgAb was examined using logarithmic regression. An equation derived from the curve that could be ordered with the best r-squared value on chart was established and used for correction of Tg levels obtained from patients with DTC who had measurable Tg (> 0.46 ng/mL) and TgAb (> 24 IU/L) levels with the assumption that the same TgAb interference exists in the presence of naturally occurring human TgAb. Differences between measured and calculated Tg levels and between L-T4 on and off Tg and TSH levels were assessed using non parametric paired test (Wilcoxon matched pairs test). A P value less than 0.05 was considered statistically significant.
For statistical analysis Statistica for Windows ’95 4.0 software package and Excel 5.0 (Microsoft) as well as SPSS for Windows (version PASW Statistics 18, USA) with 95% confidence interval were used.
Results
As shown in Table 1, there was a substantial decrease of Tg values in each of the 9 serum pools in the presence of the lowest added TgAb which increased measured TgAb levels to 39.1 ± 5.5 IU/mL. In serum pools with baseline Tg concentrations below 11 ng/mL, the addition of TgAb that increased TgAb concentration to 75.6 ± 2.5 IU/mL resulted in undetectable Tg levels, whereas Tg values became undetectable in each of the 9 serum pools in the presence of the highest concentration of TgAb.
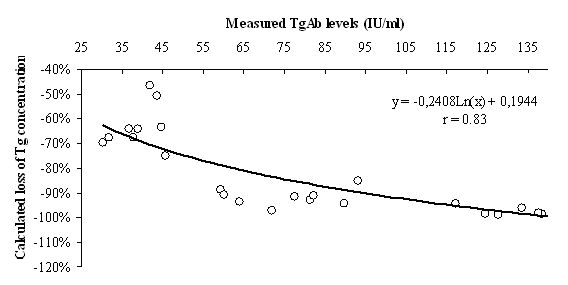
Table 2 summarises the results of regression analysis for decreasing Tg levels and loss of Tg% as depending variables. The strong relationship between TgAb and measured Tg concentrations allowed us to calculate the loss of Tg in serum pools with detectable TgAb levels (Figure 1). Changes in Tg after the addition of increasing concentrations of TgAb were described as loss of Tg% = -0.2408 x Ln(TgAb IU/mL) + 0.1944 (r = 0.83; P < 0.001).
Table 2. Summary of regression analysis for decreasing Tg levels and loss of Tg% as depending variables.
Figure 1. Nomogram for the calculation of loss of Tg%.
r - coefficient of variation.
Of the 134 serum samples obtained from patients with DTC, TgAb concentrations were undetectable (below 24 IU/mL) in 13 sera (10%), within the reference range (between 24 and 115 IU/mL) in 106 (79%) and above the upper limit of the reference range (> 115 IU/mL) in 15 samples (11%). There was no difference in follow-up times between TgAb positive and TgAb negative patients. The percent occurrence of undetectable Tg was similar in samples with TgAb levels within the reference range (56/106 samples, 52.7%) and in those with elevated TgAb levels (8/15 samples, 53%). There was a significant increase of both TSH from a median of 0.10 (0.05 1.27) to 28.3 (16.0 44.0) mIU/L and Tg concentrations from 0.09 (0.09 3.20) to 7.15 (0.09 80.00) ng/mL in serum samples obtained from DTC patients after L-T4 withdrawal (P < 0.001 for both TSH and Tg).
Of the 134 serum samples, 26 samples had detectable amounts of both Tg and TgAb (22 samples obtained during L-T4 treatment and 4 samples after L-T4 withdrawal). Using the equation defined in our in vitro experiment, calculated Tg concentrations proved to be significantly (P < 0.01) higher than measured Tg values in samples obtained during L-T4 treatment [N = 22; 5.53 (2.17-11.32) vs. 3.3 (1.6-6.9) ng/mL], but in those after L-T4 withdrawal the difference between calculated and measured Tg concentrations was not statistically significant [N = 4; 32.32 (10.1-258.1) vs. 20.4 (6.4-111.6) ng/mL]. In 2 patients calculated but not measured serum Tg exceeded 2 ng/mL during L-T4 treatment, and a marked increase of measured serum Tg after L-T4 withdrawal (16.8 and 6.1 ng/mL) in these 2 patients indicated persistence of the disease.
Discussion
Our study shows that serum TgAb concentrations within the reference range exert a significant effect on the Tg assay. We found that addition of increasing amounts of TgAb to serum pools to reach TgAb levels within or near the reference range produced a dose-dependent decrease of Tg levels. The majority of literature data propose that TgAb needs to be taken into consideration only in cases with significantly elevated TgAb levels (2,4). The potential significance of TgAb within or near to the reference range on the Tg assay has not been considered or has been neglected, although a few studies indicated that they may have an impact on Tg measurements (5-10).
Our study confirmed the frequent occurrence of TgAb in serum samples of DTC patients who underwent thyroid ablation. With the use of data obtained from our in vitro experiments with added TgAb, we were able to calculate the loss of Tg due to TgAb interference in serum samples with detectable Tg and TgAb obtained from these patients. We found that calculated Tg concentrations were significantly higher than measured values in serum samples containing detectable Tg and TgAb. In 2 patients the difference between measured and calculated Tg values was clinically remarkable and, indeed,serum Tg measured after L-T4 withdrawal indicated persisting disease in both cases. We believe that these observations may prove to be useful for the follow-up of patients with DTC. However, further studies are needed to explore the clinical relevance of our method of calculated Tg in monitoring these patients.
An obvious drawback of our study is that the equation used for calculated serum Tg can be applied only for the assays studied. Regarding the TgAb antibody used for calibration of the TgAb assay, it is not known whether sheep TgAb raised against an excess of heterologous Tg is comparable to naturally occurring human TgAb. Rosario et al. used a chemiluminescence TgAb assay calibrated against human TgAb (reference value 1 IU/mL) (7). Görges et al. also used a competitive assay and human TgAb standard (cutoff value < 50 IU/mL) (8). In contrast, our assay was calibrated using sheep TgAb and the reference range was higher than those used in these previous studies. In addition, a recent study showed marked discordance between the 4 TgAb methods studied suggesting that TgAb assays failed to predict precisely the Tg-TgAb interference (11). Inter-individual variations among TgAbs, such as distribution of immunoglobulin classes, epitope specificities and antibody avidities may substantially affect kinetics of Tg-TgAb interaction, clearance rates and biological half-lives of Tg or Tg complexed with Tg*Ab. Such differences may result in a more complex kinetics of the Tg-TgAb interaction not reflected by the equation used for calculated serum Tg in the present study. Thus, our method fails to solve the problem of Tg-TgAb interactions, but we believe that it increases the value of cohesive biochemical findings and their interpretation in routine practice.
It has been proposed that the way out of the analytical trap of determining Tg as tumour marker will probably be provided by the development of more reliable assays. In addition, several other assays have been developed to distinguish Tg produced by malignant and benign thyroid tissues, but they have not been applied for routine use (12). Until more reliable methods become available, we recommend that every laboratory should monitor TgAb interference of the used Tg method.
Acknowledgements
The authors thank Zsuzsanna Papp and technicians of the Radioimmunoassay Division for their technical support.