Introduction
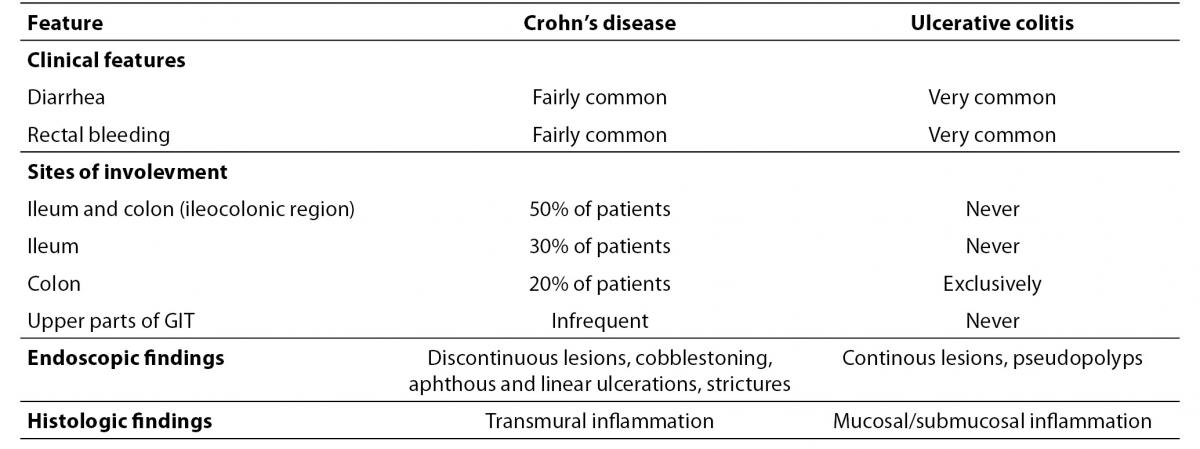
The term IBD refers to a chronic and relapsing inflammatory disorder of the gastrointestinal tract (GIT) accompanied by abdominal pain, rectal bleeding and malabsorption. It comprises two major entities, ulcerative colitis (UC) and Crohn’s disease (CD). Despite sharing similar clinical features, these diseases have significant clinical, endoscopic and histopathological differences (Table 1) (1-4).
Table 1. The key features of Crohn’s disease and ulcerative colitis.
Typically, IBD manifests between adolescence and the third decade of life, with approximately 10% of cases in individuals younger than 18 years (5). IBD diagnosis is based upon the coevaluation of clinical findings, endoscopic, radiological, histological and laboratory investigations with the main goal of excluding other conditions with similar presentations and defining the extent and severity of inflammation. In the majority of cases, endoscopic findings and histological examination of tissue biopsies provides a specific diagnosis of UC or CD (3,6-8). However, despite all available diagnostic methods, approximately 5 to 15% of patients with IBD affecting the colon are unclassifiable, as they present features of both conditions. Such patients are diagnosed with indeterminate colitis (IC) or IBD unclassified (IBDU), which is considered to be a temporary diagnosis since about 80% of these patients will eventually be diagnosed with either UC or CD (2,9,10).
The growing body of evidence suggests that IBD evolved as a result of inappropriate and ongoing activation of the mucosal immune system driven by the commensal luminal microflora in a genetically susceptible host (2,4,11-14). The triggering factor for disturbance of the tightly regulated balance between immune tolerance and defensive inflammatory response to intestinal microbiota is yet to be discovered. The serological immune response in IBD patients, which includes antibodies against the yeast Saccharomyces cerevisiae (ASCA), Escherichia coli outer membrane porin C (Omp-C), flagelin (cBir1) and Pseudomonas fluorescens – associated sequence I-2 (I2), suggests that commensal flora or a dietary antigen is the triggering factor. On the other hand, the autoimmune concept has its base in the autoimmune extraintestinal manifestations of IBD (inflammation of the skin, eyes and joints), successful immunosupressive therapy and a variety of autoantibodies including antineutrophil cytoplasmic antibodies (ANCA), antibodies against exocrine pancreas (PAB) or intestinal goblet cells (GAB) (15-17).
The objective of this review is to give an overview of the current knowledge of the serological markers in IBD with regard to their use in differentiating IBD from other conditions with similar presentation, in differentiating UC from CD, in disease stratification and prediction and, finally, their response to therapeutic interventions in IBD.
Serologic markers of IBD
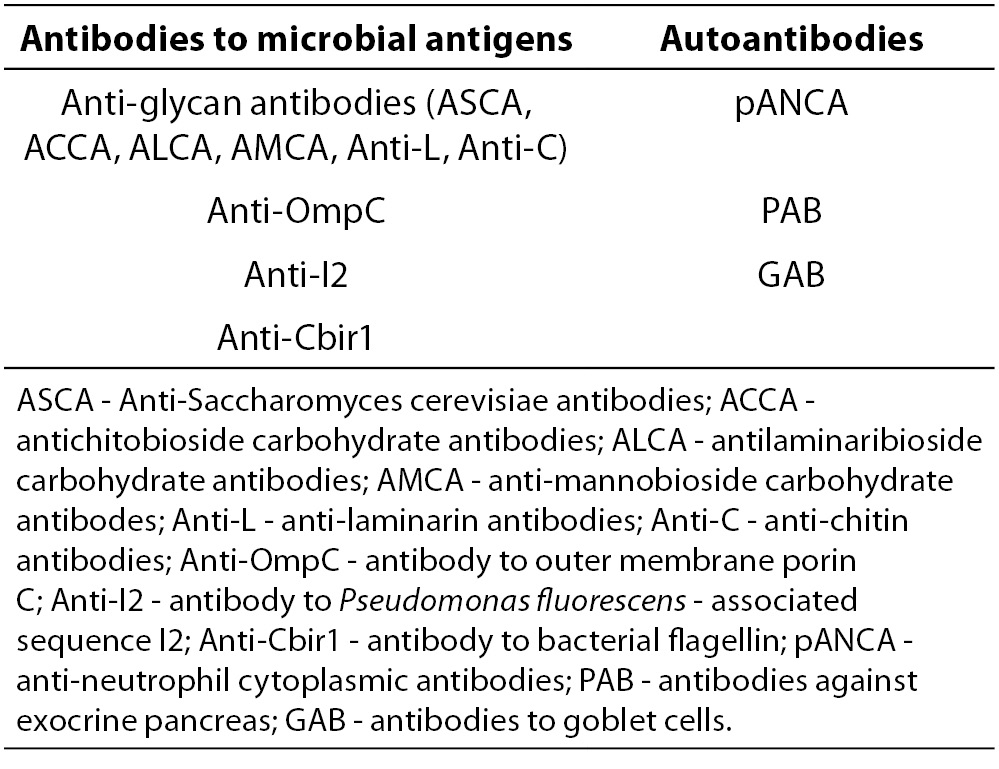
Generally, antibodies related to IBD encompasses two main groups: antibodies targeting microbial antigens and autoantibodies (Table 2.).
Table 2. Serological markers of IBD.
Anti-glycan antibodies
These antibodies targets cell wall carbohydrate epitopes found in microbiota such as yeasts and bacteria (18,19). The most prominent member of this group of antibodies are anti-Saccharomyces cerevisiae antibodies (ASCA). The major antigen targeted with ASCA antibodies is the 200 kDa phosphopeptidomannan (PPM), a cell wall mannan of the common baker’s or brewer’s yeast Saccharomyces cerevisiae. The greatest discrimination among patients with Crohn’s disease, ulcerative colitis, and controls was obtained with the Su1 strain of S. cerevisiaeused in beer brewing and mannotetraose was identified as the most important polysaccharide epitope within PPM (16,20). Regarding the widespread distribution of oligomannosides, three theories have been presented in an attempt to explain the mannose-induced immunological response. The first theory assumed that ASCA antibodies originate from immunization by dietary yeasts or yeasts that colonize the digestive tract, as a consequence of increased exposure of yeast antigens to immune reactive cells due to increased intestinal permeability (20-23). The second theory considers the epitopes shared by other microorganisms (Mycobacterium species), and the third presupposes structural homologies between S. cerevisiae oligomannosides and oligomannosides expressed on human glycoconjugates as autoantigens or neoautoantigens (20,24). ASCA was shown to have a high specificity for CD, and both IgA and IgG antibodies are formed. Methods used for the detection of these antibodies are indirect immunofluorescence (IIF) using smears of Saccharomyces cerevisiae or standardized enzyme linked immunosorbent (ELISA) assays with an antigen derived from disrupted or boiled S. cerevisiae and phosphopeptidomannan purified from the cell wall (gASCA assay) coated on microtiter plates (25-30).
In an attempt to identify novel antibodies associated with inflammatory bowel disease, Dotan et al. (18) profiled sugar-binding antibodies from the serum of patients with diagnosed CD or UC using glycan array technology and ELISA. The newly identified antibodies were antilaminaribioside carbohydrate IgG antibodies (ALCA), antichitobioside carbohydrate IgA antibodies (ACCA) and anti-mannobioside carbohydrate IgG antibodes (AMCA) (18,31,32). Laminaribioside is a building block of the glucose-based glycan laminarin, while chitobioside is a building block of the N-acetyl-glucosamine-based glycan chitin. Both laminaribioside and chitobioside, as well as mannose and mannan, are components of the cell walls of microorganisms such as bacteria, fungi and yeast and are capable of stimulating the immune system, specifically innate immunity (33). The most recently discovered members of the anti-glycan family of antibodies in IBD were anti-laminarin (anti-L) IgA antibodies and anti-chitin (anti-C) IgA antibodies (34). Similarly to ASCA, these antibodies have proven to be specific for CD, though with significantly lower sensitivity.
Antibody to outer membrane porin (anti-OmpC)
OmpC is an outer membrane porin C isolated from Escherichia coli. Originally, this protein was identified as a pANCA cross-reactive antigen using the library of colonic bacteria (35). The ELISA assay demonstrated an excessive secretion of IgA anti-OmpC antibodies in CD patients (36,37).
Antibody to Pseudomonas fluorescens - associated sequence I2 (anti-I2)
In 2000, the novel DNA sequence (I2) with homology to the ptxR and tetR bacterial transcription factor family was isolated from CD colonic lesional mucosa, suggesting that the microorganism expressing the I2 gene product may be related to CD pathogenesis (38). This bacterial sequence has been shown to derive from Pseudomonas fluorescens (39). An ELISA assay showed frequent immunoglobulin A seroreactivity in CD as opposed to UC or other inflammatory enteric diseases and healthy individuals (37,38).
Antibody to bacterial flagellin CBir1 (anti-CBir1)
Among bacterial antigens, flagellin is an interesting candidate to play a role in mucosal immune responses because it is a common bacterial antigen present on most motile bacteria in the gut and is highly antigenic. Multiple strains of colitic mice had elevated serum anti-flagellin IgG2a responses, and flagellin CBir1 has been identified as an immunodominant colitogenic antigen. In line with this, it has high anti-CBir1 IgG reactivity in human CD patient sera, as detected with the ELISA assay, and only minor reactivity in the sera of patients with UC or other inflammatory GIT diseases. CBir1 flagellin is most closely related to the flagellins of bacteria in the genera Butyrivibrio, Rosburia, Thermotoga, and Clostridium and fall within the Clostridium subphylum XIVa cluster of Gram-positive bacteria (40).
Anti-neutrophil cytoplasmic antibodies (ANCA)
ANCAs are classically associated with small-vessel systemic vasculitis such as Wegener granulomatosis, Churg-Strauss syndrome, microscopic polyangiitis and its renal-limited variant (pauci-immune necrotizing and crescentic glomerulonephritis), where their measurement is used in the purposes of diagnosis, prognosis and in monitoring of inflammatory activity (41). In vasculitis, this heterogenous family of antibodies targets different proteins, mainly located in the azurophilic granules of neutrophils and in lysosomes of monocytes. According to the International Consensus Statement (41), ANCAs are screened by indirect immunofluorescence (IIF) on normal peripheral blood neutrophils where two main types of fluorescence pattern are obtained: cytoplasmic granular with accentuation between nuclear lobes (cANCA) and fine homogenous, diffuse rim-like staining of the perinuclear cytoplasm (or rim-accentuated fluorescence of the nuclei) designated as the pANCA pattern. The major ANCA antigen targets in inflammatory vasculitides are proteinase-3, mainly displaying cANCA pattern and myeloperoxidase associated with the pANCA pattern (41-43).
The distinct subset of ANCA associated with UC was first reported in 1990 (44,45). The pattern of staining on IIF exhibited broad inhomogeneous rim-like staining of the nuclear periphery, different from classical pANCA and was designated as atypical p-ANCA. Various target antigens of atypical pANCA have been intensively studied in IBD patients. These studies included proteins located in the granules of the neutrophils and monocytes such as: serine proteases cathepsin G and elastase, hydrolase b-glucuronidase, iron-binding protein lactoferrin and the natural antibiotic bactericidal permeability increasing protein (BPI); cytoplasmic proteins such as a-enolase and catalase; proteins distributed in the cytoplasm and nuclei of eukaryotic cells: high-mobility group of non-histone chromosomal proteins (HMG-1 and HMG-2), and finally proteins located in the nuclei, such as histone H1 (25,27). Overall, most studies supported the conclusion that IBD-associated ANCA specific antigens are not located within neutrophil granulas but rather within the nuclei. The immunoelectron microscopy finding of UC-associated pANCA reactivity localized over chromatin concentrated toward the periphery of the nuclei supports this thesis (46). However, UC sera did not react with double-stranded DNA. Vidrich et al. (47) demonstrated the loss of the UC-related pANCA staining pattern after digestion of substrate cells with DNAse, suggesting that the epitope recognized by this subset of antibodies is a protein-DNA complex or that the presence of intact DNA is necessary to maintain the integrity of the epitope. It is likely that the target antigen for UC-related atypical pANCA is a complex conformational epitope which comprises the previously reported nuclear proteins histone H1, HMG-1 and HMG-2. Since the target antigen for UC-associated pANCA is yet unrecognized, sensitive and specific solid-phase methods cannot be developed, and therefore IIF on normal peripheral blood neutrophils is still the most commonly employed method in use. Detection of DNase I - sensitive pANCA antibody is more specific for UC in differentiation from similar pANCA patterns in autoimmune liver diseases. Recently, lactoferrin was suggested as a major pANCA target in UC but it has to be bound to DNA to present epitope relevant for the reaction with the autoantibodies. Use of the lactoferrin reconstituted (LFR) granulocytes (granulocytes stripped of pANCA targets and then reconstituted with human lactoferrin) as a substrate in addition to standard ethanol-fixed granulocytes raised the sensitivity of the IIF assay from 71.8% to 87.2% (48).
Antibodies against exocrine pancreas (PAB)
These antibodies were first described in 1987 by Stöcker et al. (49) who tested sera of patients with CD and UC for autoantibodies by the IIF method using 19 different human tissues as antigenic substrates. They demonstrated that autoantibodies against exocrine pancreas (PAB) were found almost exclusively in CD patients (although with rather low sensitivity) and suggested that this specific autoantigen is a component of normal pancreatic juice. In the IIF assay on sections of human or primate pancreatic tissue, PAB antibodies stain different structures of the exocrine pancreas and are divided into two subtypes accordingly: subtype I with a typical, extracellular, drop-like staining pattern in the acinar lumen of pancreatic tissue sections, and subtype II with speckled staining of the cytoplasm of pancreatic acinar cells (50). Recently, the major zymogen granule membrane glycoprotein (GP2), a glycosylphosphatidylinositol (GPI)-anchored protein of the pancreatic acinar cells was identified as the autoantigen of PAB in CD (51). Upon hormonal or neuronal stimulation of the pancreas, GP2 is transported to the apical compartment of acinar cells, from which it is released, together with zymogens, into the pancreatic duct. The two obtained fluorescence patterns of PAB on pancreatic tissue are consistent with the localization of GP2. In addition to pancreatic acinar cells, M cell-specific expression of GP2 in humans and mice was also seen among the intestinal epithelium (52,53) The characteristic of the M-cells as specialized epithelial cells of mucosa-associated lymphoid tissues (Peyer’s patches) is their role in the transport of antigens from the lumen to the cells of the immune system (54). It was found that the GP2 expressed on M cells serves as an uptake receptor for a subset of commensal and pathogenic bacteria (53). Bearing in mind that Peyers patches are abundant in the distal part of the ileum, the predominant site of inflammatory onset in CD, Roggenbuck et al. (51) demonstrated GP2 expression at mRNA and protein levels in colon biopsies from patients with CD at a significantly higher level than in UC colon biopsies. This observation supports the hypothesis of a direct involvement of anti-GP2 in CD pathophysiology, rather than being merely an epiphenomenon as an antibody targeting non-intestinal antigen.
Antibodies to goblet cells (GAB)
Intestinal epithelial cells represent a physical barrier against the excessive entry of bacteria and other antigens from the intestinal lumen into the circulation. Goblet cells, as specialized intestinal epithelial cells, regulate the production of mucus and factors that contribute to epithelial repair and regulation of inflammation (4). GAB have been detected primarily in adult UC patients with prevalence varying from 15% to 46.6% while in CD patients observed prevalence of GAB showed even wider range from 1.4% to 33% (15,49,55,56). Obtained differences in GAB prevalence are likely attributed to methodological differences, such as the use of different origin of antigenic substrate for IIF: human or monkey intestinal tissue, rarely rat jejunum or human colonic cancer cell line HT29-18-N2, which differentiates into intestinal goblet cells. Tissue substrates are associated with problematic reproducibility due to the natural fluctuations of tissue quality. The next sources of variability in the obtained results lie in the evaluation of fluorescence patterns on IIF. Beside the IIF test, the ELISA assay using the HT29-18-N2 cell line assay is also in use for GAB detection (55).
Clinical usefulness of serological investigation of IBD
The main concerns regarding clinical usefulness of serological markers in IBD refers to: a) their efficiency in distinguishing IBD from other diseases with similar clinical presentation and in distinguishing subtypes of IBD (ulcerative colitis from Crohn’s disease), b) their prognostic value in stratifying disease phenotypes, and c) monitoring disease activity and reflecting the response to therapeutic intervention.
Serological investigation for IBD diagnostic purposes
Use of serological markers in distinguishing IBD from other non-IBD gastrointestinal diseases
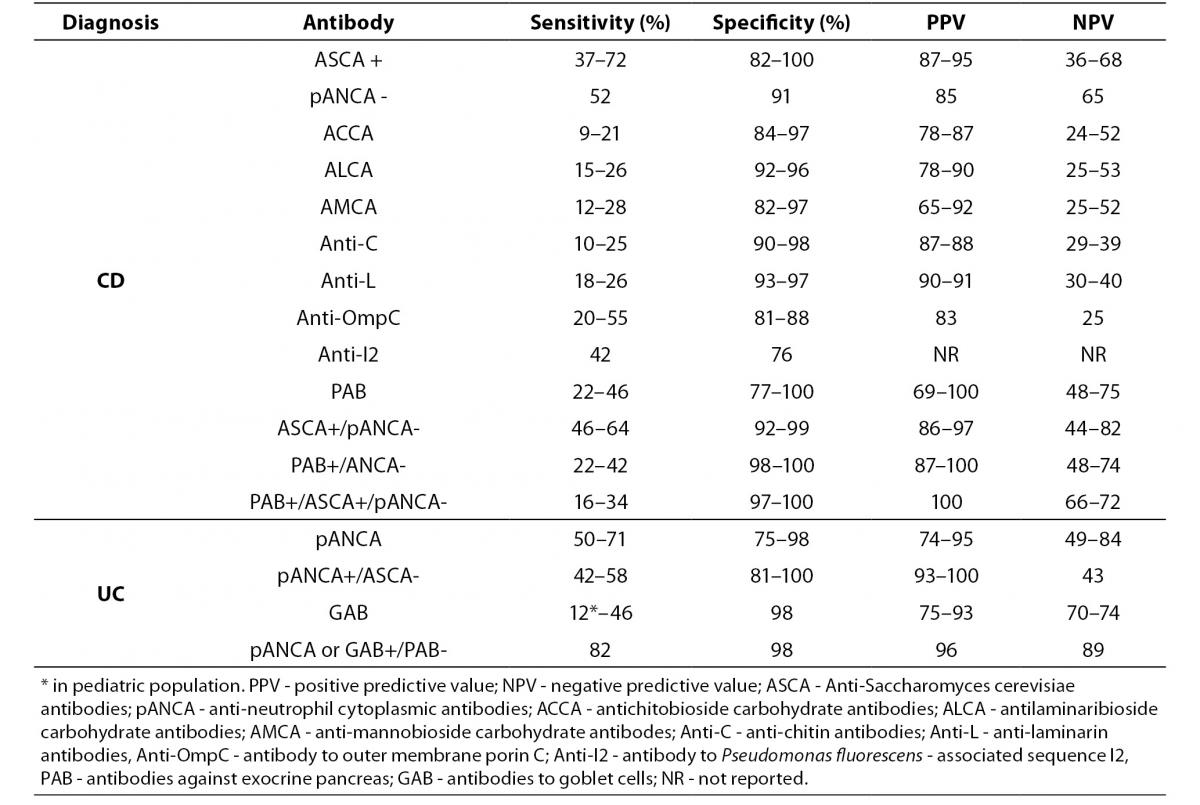
Currently, diagnosis of IBD is based on a combination of clinical, radiological, endoscopic and histological studies and, in most cases, the diagnosis can be made with high certainty. Assessment of the currently known IBD-associated antibodies has not surpassed the diagnostic accuracy of the previously mentioned conventional methods, mainly due to their limited sensitivity (Table 3) (15,19,26,27, 29,33,55,56-59).
Table 3. Prevalence of individual serological markers in patients with IBD, non-IBD GIT disorders and healthy individuals (15,19,26, 27,29,33,55-59).
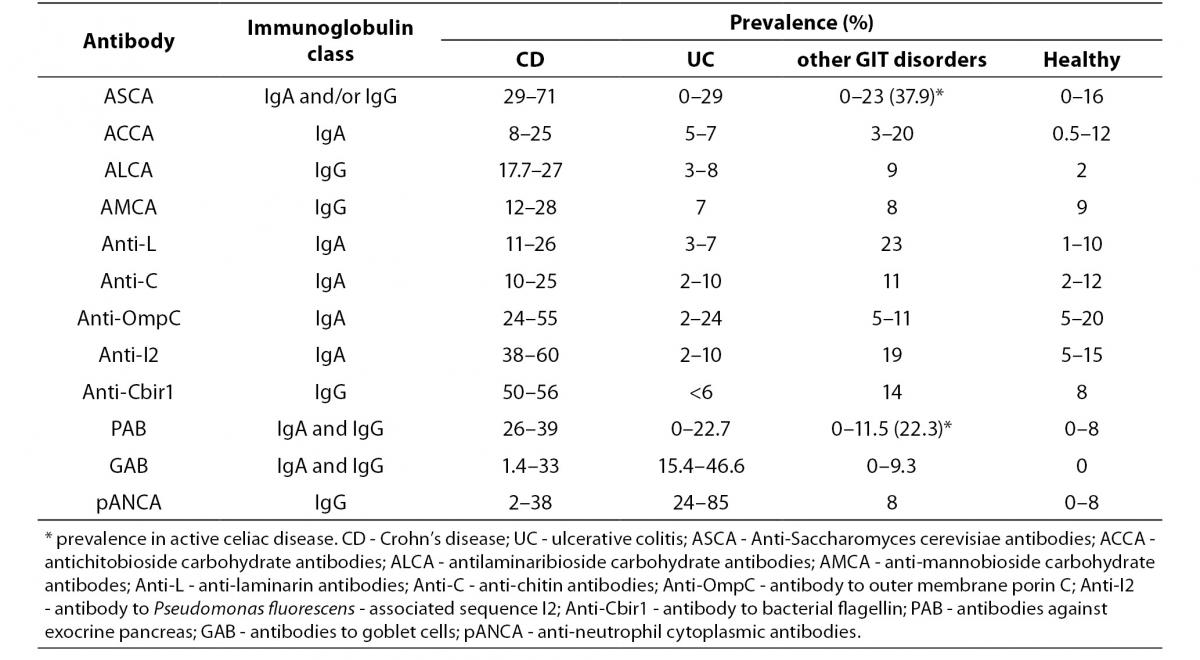
A positive test result for any individual antibody, even those with highest sensitivity like pANCA or ASCA, only modestly influences the pretest/posttest probability in distinguishing IBD from other GIT disorders with similar clinical presentation, while a negative test result has no clinical value. Although most studies have confirmed the high specificity of IBD-associated antibodies (75–99%), caution should be taken in considering the control groups of non-IBD GIT disorders that mainly included irritable bowel syndrome, infectious colitis or functional gut disorders (26). Namely, ASCA that was considered as a highly CD-specific antibody was observed in 30% to even 59% of patients with celiac disease prior introducing the gluten-free diet. ASCA antibodies were more often of the IgG class and were, unlike the ASCA-IgA, insensitive to gluten withdrawal (60,61). Another highly CD-specific antibody, PAB, showed a frequency of 22.3% in celiac patients at diagnosis (more often IgA class), and positivity demonstrated a tendency to be lower in patients on a strict gluten-free diet (56). This observation suggests that the presence of ASCA or PAB may be a marker for increased permeability of the small bowel and autoimmunity instead of a specific IBD (CD) marker.
Therefore, at the present, assessment of serological markers are not suitable for screening for IBD in patients with gastrointestinal symptoms, but rather as assistance in cases of a diagnostic dilemma.
The variation in the prevalence of individual serological markers across studies could be explained by differences in the methods used. For example, pANCA have been determined with standardized IIF on ethanol fixed neutrophils from healthy donors, or with fixed granulocytes ELISA, or using ELISA followed with IIF for ANCA-ELISA positive samples. Some studies were performed with IIF including the step with DNase I digestion of neutrophils. Variation in the sensitivity of pANCA assays from 0–63% in UC samples across five different laboratories suggests that these assays obviously do not detect the same spectrum of antigens (28). Also, considerable lack of agreement exists within the same methodology due to differences in antigen preparation and quality, cut-off values based on the receiver operating curve, differences in evaluation of fluorescence patterns for IIF methods, cut-off titer or the origin of the substrate used for IIF.
Distinguishing CD from UC
The heterogeneity within the IBD group of disorders influences the clinical presentation with overlapping symptoms of CD and UC. In up to 15% of cases, no differentiation into a particular IBD subtype can be made, giving rise to a diagnosis of IBD-unclassified (IBD-U) or previously known as indetermined colitis (IC). Differentiation into either of the IBD subtypes in the early phase of the disease has an influence on the tailoring of drug therapy. According to retrospective database analysis of 250 children diagnosed with IBD, IBD-U appears to have a higher prevalence among paediatric patients (up to 29.6%) and is associated with early disease onset and rapidly progresses to pancolitis (62). In the same study, 66.2% patients maintained their diagnosis of IBD-U after a mean follow-up of 7 years, which favours the hypothesis of some investigators that IBD-U is a unique disease phenotype within the IBD group, with more a extensive disease, more severe clinical course and higher rate of complications. Paediatric IBD patients comprise a particular population in whom non-invasive testing is desirable and who would benefit the most from the early proper therapeutic approach. As the specificity of serological markers exceeds their sensitivity, serological profiles can be useful in the differentiation of IBD subtypes (62,63).
Most of the data pertaining to the usefulness of serological markers in distinguishing CD from UC refer to the ASCA and pANCA antibodies, while fewer data are available for other anti-glycan antibodies, anti-I2, anti-Omp-C, anti-Cbir1, PAB or GAB. Overall, ASCA has the best combined sensitivity and specificity for CD and pANCA for UC. Most other investigated serological markers are specific for CD, with the exception of GAB. Table 4. summarizes the data on diagnostic accuracy of individual and combined antibodies in the differential diagnosis of of CD and UC (10,15,26-30,56,64-66).
Table 4. Diagnostic accuracy of individual serological markers and their combinations in differential diagnosis of CD and UC (10,15,26-30,56,64-66).
The general opinion is that combined testing instead of individual antibodies is more useful in obtaining a differential diagnosis of CD versus UC. Profile ASCA+/pANCA- increases specificity and positive predictive value (PPV) for diagnosis of CD comparing to ASCA+ as isolated result. In the same manner, the reverse profile pANCA+/ASCA- was shown to have a higher specificity and PPV for diagnosis of UC than pANCA+ alone (26-30). Profile pANCA-/ASCA- was found to have a strong positive correlation with IBD-U diagnosis. Prospective study of IBD-U patients revealed that half of the patients had pANCA-/ASCA- seroprofile and the vast majority of them did not change the initial diagnosis after 6 years. Therefore, pANCA-/ASCA- seems to be the serological marker closely associated with IBD-U as a separate disease entity (30,63).
Recent study assessed the reactivity of seven anti-glycan antibodies in a large cohort of 818 IBD patients, including gASCA IgA and IgG (gASCA is an improved ASCA assay based on immobilized purified mannan polysaccharide), ACCA, ALCA, AMCA, anti-L and anti-C (34). Within the CD patient population, 73% were positive for ³ 1 anti-glycan antibody. All anti-glycan markers were specific for CD and were significantly more prevalent in CD than in UC. The most efficient discrimination between CD and UC was achieved by the addition of anti-L and anti-C to gASCA/pANCA panel while adding of anti-L to the same panel improved differentiation of colonic CD from UC.
Diagnostic accuracy for CD versus UC of the gASCA and ALCA antibodies was find out to be similar between adult and paediatric IBD cohorts, while discrepancies were found for AMCA and ACCA. In paediatric population, both serologic markers showed significantly higher specificity, while AMCA showed significantly lower sensitivity compared to adults (33).
The importance of combined testing was pointed out by the finding that about one third of ASCA negative CD patients may be positive for at least one of the previously mentioned anti-glycan antibodies (67).
Similarly, anti-CBir1 were positive in about half of ASCA-negative adult CD patients while the addition of the anti-CBir1 assay to the ASCA, pANCA and anti-OmpC panel halved the number of serologically negative paediatric CD patients (68,69).
PAB was confirmed as a highly specific marker of CD in several studies, but with low sensitivity (15,29,65). Therefore, the use of PAB in combination with pANCA and ASCA was suggested, particularly in the differentiation between isolated colonic CD and UC where the clinical difficulty lies in the differentiation between CD and UC. In another study, authors concluded that PAB detection could be useful only in clinically highly suspected patients without circulating ASCA, since they found PAB positivity in 14% of CD patients that were negative for ASCA (29). Several studies, however, showed lower specificity of PAB in distinguishing CD from UC both in adult and paediatric patients (56,63,66).
In majority of reports, GAB was confirmed as highly specific serological marker in distinguishing UC from CD, but due to the low sensitivity (especially in paediatric population, 12%) it is poorly usable in differential diagnosis of IBD subtypes, both in adults and children (15,56,65,66,70).
However, in a recent study by Homsak et al. (15), the combination of positive pANCA or GAB with negative PAB managed to detect the majority of UC patients.
In summary, testing for isolated antibody is of limited value in differential diagnosis of IBD subtypes, while the combined testing of several antibodies (serologic panels) significantly improves specificity and PPV for certain IBD subtype. Furthermore, widening panel of antibodies can also improve the sensitivity. However, result of serological testing is not decisive but is an adjunctive tool in patients in whom all other clinical features does not allow a distinction between CD and UC.
Use of serological testing in disease phenotype stratification
The heterogenic nature of both CD and UC is reflected in the different phenotypes of the disease, according to location, clinical course and activity or behaviour patterns, and response to treatment within each IBD subtype. An ability to stratify IBD subtypes by the risk for disease progression and complications would most certainly improve overall disease outcomes through an early decision of the most appropriate treatment option available.
In addition to contributing to an improved IBD diagnosis, there is mounting evidence of a link between serum immune reactivity and specific clinical phenotypes in IBD.
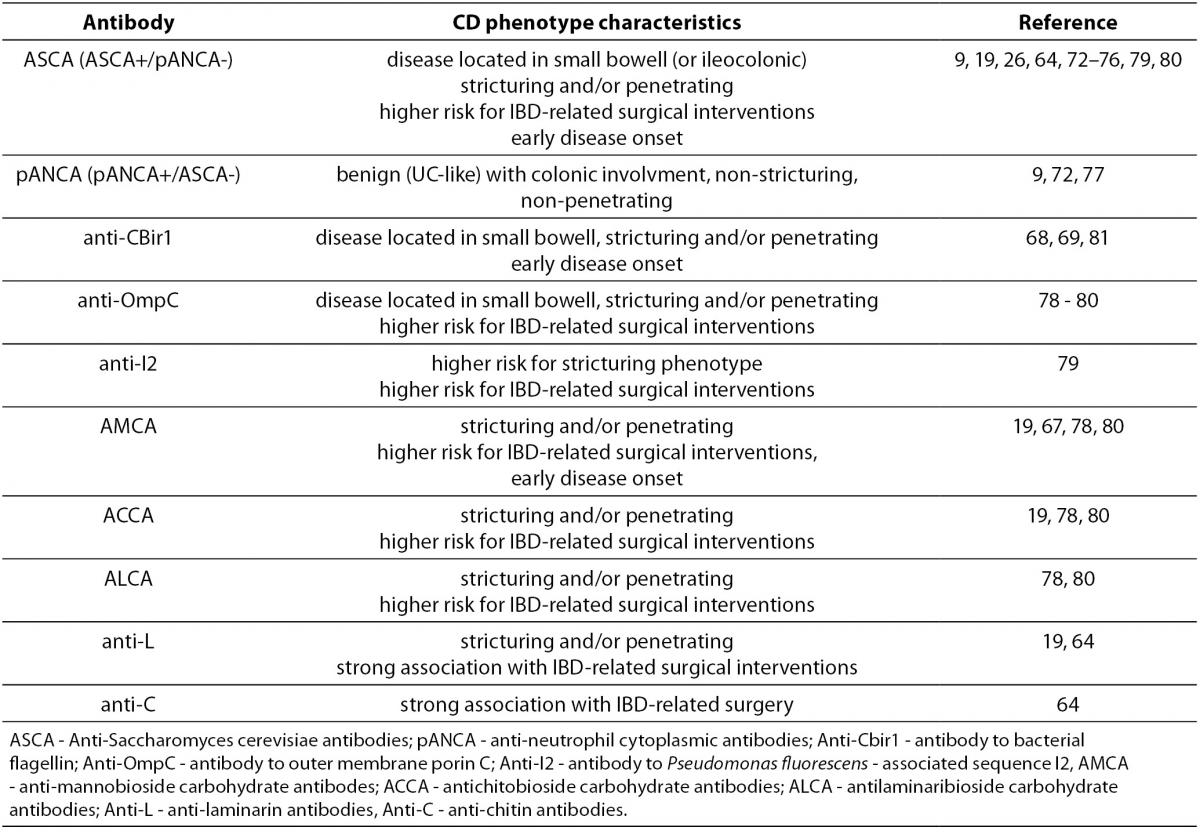
In CD, disease extent can evolve with time from a non-stricturing, non-fistulizing, inflammatory phenotype to a more severe stricturing (fibrostenotic) or penetrating (with internal fistulae, fistulizing) phenotype (71). Numerous studies have examined the presence of different IBD-related antibodies and disease behaviour (9,19,26,64,68,69,72-80). Positive association of the most of the anti-microbial, especially anti-glycan antibodies with more complicated CD phenotype and a higher frequency of Crohn’s disease-related abdominal surgery has been consistently demonstrated (Table 5). It is important to emphasize that this association becomes stronger with increasing diversity (multiple antibodies) and magnitude (higher titers) of the serologic response.
Table 5. Association of serological markers with CD phenotype.
Similar results were obtained in the paediatric population. Dubinsky et al. (81) evaluated associations between anti-I2, anti-OmpC, anti-CBir1 and ASCA immune response and clinical phenotype in 196 paediatric CD patients. Increased frequency of internal penetrating and/or stricturing disease with increasing diversity of immune response was demonstrated, with the highest odds in patients positive for all four antibodies. Also, patients positive for ³1 antibody progressed to a complicated disease faster than those negative for all antibodies. These results were further confirmed for the same panel of antibodies in a later study performed on a 796 paediatric CD patients (82).
Data on the association of PAB with the CD phenotype are somewhat conflicting. In the Eastern European IBD cohort, antibody response to PAB was proven to be associated with complicated disease phenotype and extraintestinal manifestations. The presence of PAB was associated with perianal disease and extraintestinal manifestations such as arthritis, ocular or cutaneous manifestations. Also, the presence of PAB IgA antibodies was associated with penetrating disease behaviour (56). On the contrary, in the study of Joosens et al. (57) both PAB patterns were negatively associated with stricturing disease behaviour of CD. In the study conducted on Caucasian and Chinese IBD populations, PAB expression was not associated with stricturing or perforating CD, while in another study, the small differences in PAB prevalence in CD subtypes do not suggest that PAB detection is useful in the discrimination of CD phenotypes (65,83). In a study of PAB and GAB antibodies in paediatric IBD patients, there was a lack of correlation with the clinical phenotype (66).
In contrast to CD, UC has a less heterogeneous disease behaviour but can evolve into a more aggressive phenotype with regard to a higher number of surgical interventions, moderate to severe disease activity or larger disease extent. In the study performed on 366 IBD patients, no relation of pANCA in UC patients or in CD patients was found with disease activity, duration of illness, location, disease extent, previous bowel operations or medical treatment (84). In another study, a tendency of higher prevalence of pANCA+ or pANCA+/ASCA- reactivity in severe UC compared to remission cases according to the Montreal classification was demonstrated, though this difference was not statistically significant. The pANCA-/ASCA- pattern was observed less often in active UC when compared to remission phase although with borderline significance (9).
In other UC cohorts, pANCA expression was significantly associated with a higher relapse rate, more aggressive disease course requiring early colectomy or with the treatment-resistant left-sided disease (85-87). A possible association between pANCA and relative resistance to medical therapy in UC patients was also recently documented, with negative pANCA status as an independent positive predictor for response to treatment with infliximab (88).
Recent studies indicated that serological responses may identify patients with higher risk for postoperative complications in patients with UC or IBD-U. Patients who were pANCA-/ASCA+ were shown to have an increased risk for the development of fistulas after surgical intervention compared to patients who were pANCA+/ASCA-, and were also more likely to have their diagnosis changed postoperatively to CD (89). Another study confirmed preoperative ASCA-IgA seropositivity as a predictor of postoperative CD diagnosis in UC and IBD-U patients (90). There is an indication that high levels of pANCA prior to colectomy is significantly associated with the development of postoperative complications in UC patients (91).
In summary, an assessment of serological markers is useful as a predictor of complicated disease behaviour in CD or in predicting postoperative complications in UC or IBD-U patients. The presence of multiple antibodies and the magnitude of the immunological response appear to be the strongest predictors of disease progression.
Use of serologic markers in monitoring disease activity and response to drug therapy
According to the available data, there is no use of serial measurement of IBD serological markers, including ASCA, ALCA, ACCA, anti-OmpC or pANCA in monitoring disease activity (26,27,92).
Regarding the association with response to therapy, there was no relationship between ASCA or pANCA and response to therapy in a study conducted on 279 CD patients before starting anti-TNF therapy (infliximab). Although lower response rates were observed for patients with refractory intestinal disease carrying the pANCA+/ASCA- combination, this finding lacked significance (P = 0.067) (93). Possible association of pANCA with relative resistance to medical therapy was further documented in a study by Sandborn et al. (87) who found an increased frequency of pANCA in treatment-resistant left-sided ulcerative colitis. The report of a negative pANCA status as an independent positive predictor for response to therapy with infliximab in UC patients supports this (88). Other studies have not found an association between ALCA, ACCA, AMCA, anti-OmpC, anti-I2 or pANCA and treatment in CD (26).
Other aspects of IBD serological markers
Prevalence and diagnostic value of IBD serological markers have shown significant variation among different ethnic or geographic population. For example, in Chinese, Japanese and Iranian CD patients ASCA was shown to be less sensitive comparing to Caucasians. On the other hand, studies conducted on Tunisian, Korean and Brasilian patients yielded prevalences comparable to Caucasian CD patients The prevalence of pANCA was found to be lower in Chinese, Japanese, Korean, Thai and Romanian patients with UC but higher in Mexican-American compared to Caucasian UC patients (26,32). Therefore, these data suggest that ethnic background should be considered when applying IBD serological markers in clinical practice.
Family studies indicated ASCA as potential subclinical biomarker for population in risk for CD, as it has been reported that this antibody is present with significantly higher frequency (20-25%) in unaffected first-degree relatives of CD patients, as compared to general healthy population (0-10%) (94,95). Furthermore, in the retrospective study, ASCA reactivity was found at a median of 38 months before clinical diagnosis in 32% of CD patients (96). In contrast to ASCA, PAB seems to rarely occur in family members of patients with Crohn’s disease (50).
pANCA was not proven as a marker of increased susceptibility for disease in first-degree relatives of patients with UC (26). Recent study assessed risk factors for CD in multicase families and a cumulative effect of number of first-degree affected relatives, and number of positive antimicrobial antibodies (ASCA, AMCA, ALCA, ACCA, anti-OmpC, anti-CBir1, Anti-I2) was found (97).
An interesting aspect of IBD-related serologic response is their possible role in bridging the genetic susceptibility and clinical disease. Several studies investigated the association of serological markers with IBD-susceptible gene mutations (26,98). In spite of some inconsistency, more studies found ASCA frequency significantly associated with greater frequency of mutant NOD2/CARD15 alleles and also the genotype-seroreactivity synergism in predicting complicated CD phenotypes (33,59, 72,99). In accordance with this, it is reported that association of other anti-glycan antibodies (ALCA, AMCA, ACCA and ASCA) with NOD/CARD15 mutations in a dose-effect manner is found where more mutations were associated with higher seroreactivity (78,80).
Conclusions
The current diagnostic approach based upon clinical, endoscopic, histological, radiological and biochemical criteria provides a reliable diagnosis in the majority of cases of IBD over other GIT disorders that share similar clinical presentation, as well as differentiation into IBD-subtypes, CD or UC. However, there are certain cases where a significant overlap is present in the results of conventional diagnostic tests, thereby makes differentiation of these two subtypes difficult. It is this particular point in the diagnostic algorithm of IBD where serological testing has the greatest benefit. Due to their lack of sensitivity, serological markers are not advisable for use in the diagnosis of IBD but rather in differentiating CD from UC, particularly with the use of a wide panel of antibodies. According to the growing evidence of an association between the magnitude of serological immune reactivity and specific clinical phenotypes, the most important clinical utility of serological markers could be in stratifying patients according to risk for aggressive disease phenotype or postoperative complications. Such a “risk score” that would integrate markers of immune response, genetic markers and clinical characteristics might enable the application of personally-tailored therapeutic strategies and better surveillance of patients at risk. At the current time, there is insufficient evidence of usefulness of serological markers in monitoring the treatment of IBD patients.