Evaluation of sample hemolysis in blood collected by S-Monovette® using vacuum or aspiration mode
Giuseppe Lippi
[*]
[1]
Paola Avanzini
[1]
Roberta Musa
[1]
Franca Sandei
[1]
Rosalia Aloe
[1]
Gianfranco Cervellin
[2]
Introduction
Total quality in laboratory diagnostics is a challenging enterprise, which requires high degrees of safety, standardization and thoughtful monitoring of all activities of testing process (1). Several lines of evidence attest that preanalytical variables play a prevailing role in decreasing the quality of testing, and contextually increase the likelihood of diagnostic errors (2-4). The preanalytical phase typically entails all those activities concerning patient preparation before testing, collection, handling, transportation and preparation of biological specimens. Among these phases, blood drawing represents indeed the most vulnerable step (5), which bears the highest probability of generating low quality and/or unsuitable samples, especially when consistent criteria for complete filling (6-8), appropriate mixing (9) and suitable storage (10) of primary blood tubes are not fulfilled.
Among the various sources that may impair the quality of in vitro diagnostics, spurious hemolysis is indeed a foremost issue (11), because hemolyzed specimens represent the most prevalent cause of sample rejection in most clinical laboratories, thus causing important safety, organizational and economical drawbacks (12), as well as relational problems with the hospital wards (13) due to prudent suppression of unreliable test results (14). In vitro hemolysis may be due to a kaleidoscope of biological and technical causes, including collection from small or fragile veins, traumatic blood draw, vein missing during venipuncture, collection with unsuitable devices, incomplete filling or mixing of primary blood tubes, unsuitable conditions for specimen storage and transport, poor barrier integrity or specimen re-spun (15). The vacuum of primary blood tubes is another aspect that has been occasionally associated with a higher chance of obtaining unsuitable specimens, so that the use of traditional syringes is still regarded as a viable alternative in certain circumstances, especially in those patients with small and fragile veins, in whom the ability to control the pressure being applied inside the vein would reduce physical stress to the blood (11,15).
At variance with other commercial systems, the S-Monovette® (Sarstedt AG & Co., Nümbrecht, Germany) is a primary blood collection system that combines the “vacuum” with the “aspiration” method. In the former case, the vacuum is generated immediately prior to collection by locking the piston into the base of the S-Monovette and breaking off the plunger, so that the device can be used as a standard evacuated device. In the latter case, S-Monovette can be used more or less as a syringe, wherein the blood is directly aspirated into the tube by slow withdrawn of the plunger until the tube is filled (Figure 1). At variance with a syringe, however, the plunger is broken off at the end of the collection process and the S-Monovette can hence be used a standard, primary blood collection tube, with no need of blood transfer from the syringe to a secondary tube. According to manufacturer’s claim, the slow manual aspiration technique may produce certain quality advantages, especially in patients with difficult vein accesses (e.g., infants, chemotherapy or geriatric patients), wherein the chance of obtaining hemolyzed specimens is higher, and it is possibly worsened by forced aspiration of blood by the vacuum (15). Therefore, the aim of this study was to compare the probability of hemolysis by drawing blood with a commercial evacuated blood collection tube, and S-Monovette used in either “vacuum” or “aspiration” mode.
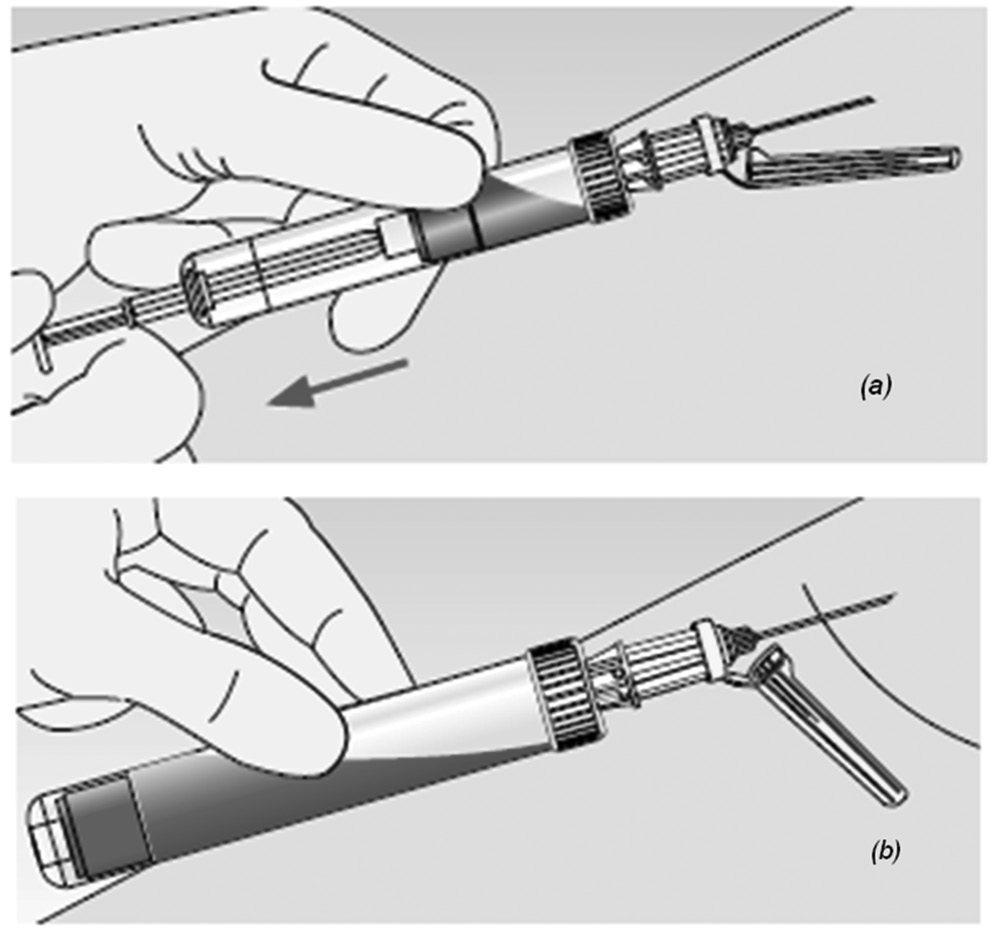
Figure 1. Blood collection with s-Monovette in aspiration (a) and vacuum (b) mode.
Materials and methods
Methods
The study population consisted in 20 healthy volunteers (11 females and 9 males; median age: 47 years; range 25 to 59 years) recruited from the staff of the laboratory. A sample labeled as “A” was drawn by a 21 gauge straight needle into a 4.0 mL BD Vacutainer® standard serum tube without gel separator (Becton Dickinson Italia S.p.A., Milan, Italy, ref. n. 369032, Lot n. 1290762), after a first identical BD Vacutainer tube had been collected and discarded, from the median cubital or basilic vein of left arm. The samples labeled as “B” (collected by vacuum) and “C” (collected by manual aspiration) were then drawn by a 21 gauge straight needle with a second venipuncture from the median cubital or basilica vein of right arm into 4.0 mL S-Monovette serum tubes without gel separator (Sarstedt AG & Co, ref. n. 04.1924, lot n. 2213501), after a first S-Monovette serum tube had been drawn with vacuum and discarded. After the first group of 10 patients, the protocol was reversed; samples “B” and “C” were collected from a vein of left harm, whereas sample “A” was collected from a vein of right harm. Blood was drawn in the morning of the same day on fasted volunteers, always by the same experienced phlebotomist (GL). All blood tubes were filled up to the nominal volume and all phases of sample collection were accurately standardized, including identical resting time of the volunteers (i.e., 5 min), time of tourniquet placement (i.e., <15 s), as well as the use of 21 gauge straight needles. The tourniquet was immediately released as soon as the blood began to flow into the first tube.
After collection, all samples were gently mixed by 4 to 6 time inversion, and allowed to clot for exactly 30 min. The serum was then separated by standard centrifugation at 1300 x g at room temperature, and tested for potassium, lactate dehydrogenase (LD) and hemolysis index (HI) on a Beckman Coulter DxC (Beckman Coulter Inc., Brea CA, USA), in which the concentration of cell-free hemoglobin is assessed by direct spectrophotometry and reported in semiquantitative value on a linear scale spanning from 0 (0 g/L of hemoglobin) to 10 (hemoglobin concentration from 4.5 to 5.0 g/L). In brief, the sample is diluted with a specific reagent, the absorbance is measured at 6 wavelengths and the raw data is then analyzed with underlying mathematical algorithms. A previous study showed that this technique provides highly comparable results with the reference cyanmethemoglobin assay (16). All tests were performed in duplicate, and results were finally averaged.
Statistical analysis
Test results obtained on samples “A”, “B” and “C”, which were normally distributed as verified by Kolmogorov-Smirnov, were shown as mean and 95% confidence interval (95% CI), and compared by Student’s paired t-test and Bland & Altman plots analysis. The results were also compared with the desirable quality specifications for allowable total error derived from biologic variation, i.e., ± 11.4% for LD and ± 5.8% for potassium (17). Each volunteer provided a written consent for being enrolled in the study, which was performed in agreement with the Declaration of Helsinki and under the terms of all relevant local legislation.
Results
The main findings of this study are shown in table 1. As regards the concentration of cell-free hemoglobin in the serum specimens, in no case the HI exceed the traditional limit of analytically and clinically significant hemolysis, that is 0.5 g/L. As compared with BD Vacutainer, no significant differences of potassium and LD were observed using S-Monovette with vacuum method. A consistent and highly significant trend towards higher values of both parameters was however observed when blood was collected into BD Vacutainer and S-Monovette by vacuum mode as compared with blood drawn by S-Monovette in aspiration mode (Table 1). In Bland & Altman plots analysis, the mean bias of potassium was 2.2% (95% CI, 0.6 to 3.8%) versus BD Vacutainer and 2.4% (95% CI, 0.5 to 4.3%) versus S-Monovette in vacuum mode. The mean bias of LD was 2.7% (95% CI, 1.5 to 4.0%) versus BD Vacutainer and 2.1% (95% CI, 0.6 to 3.7%) versus S-Monovette in vacuum mode (Figure 2). In all cases the observed variations were however comprised within the desirable specification for allowable total error for both parameters (i.e., ± 11.4% for LD and ± 5.8% for potassium, respectively).
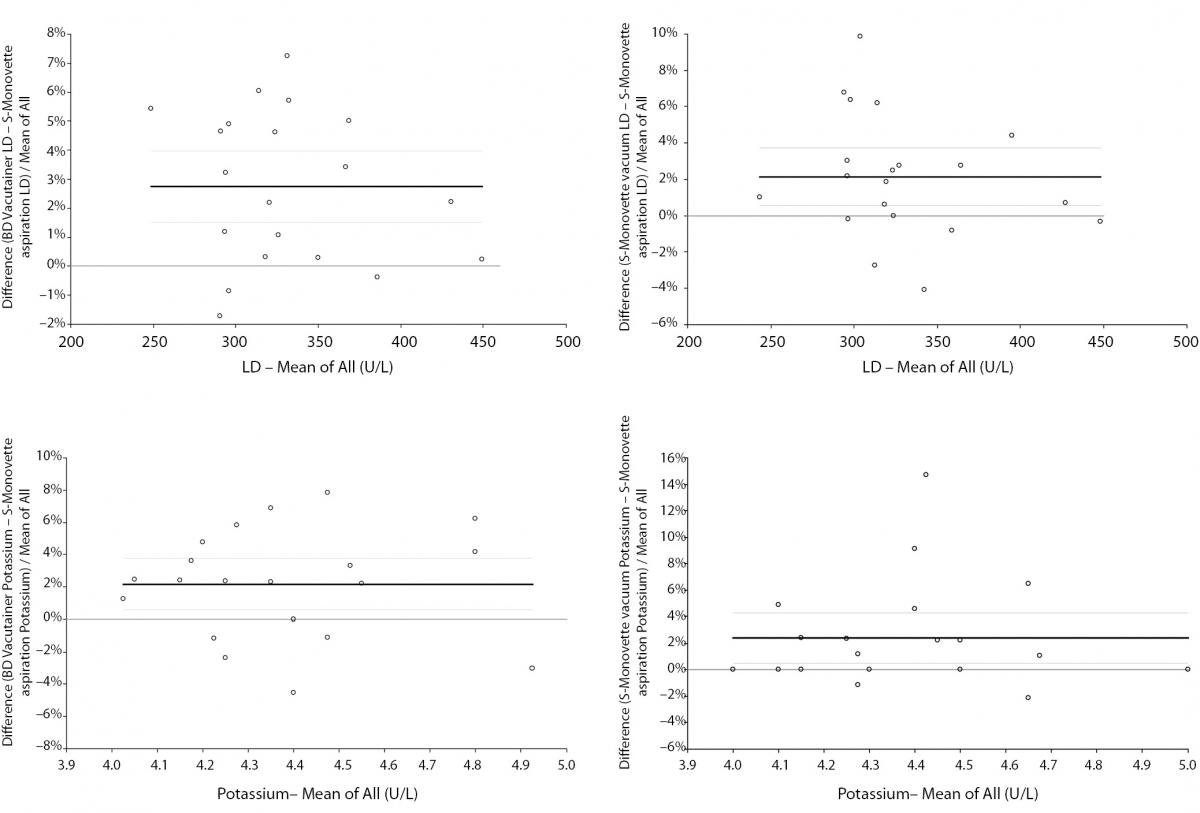
Figure 2. Bland-Altman plots of potassium and lactate dehydrogenase in serum specimens drawn into 4.0 mL BD Vacutainer® standard serum tube without gel separator and 4.0 mL S-Monovette serum tubes without gel separator used either in “vacuum” or “aspiration” mode. Solid lines are drawn at the mean difference, whereas dashed lines define the 95% Confidence Interval (95% CI).
Table 1. Values of potassium and lactate dehydrogenase (LD) (mean and 95% Confidence Interval) in serum specimens drawn into 4.0 mL BD Vacutainer® standard serum tube without gel separator and 4.0 mL S-Monovette serum tubes without gel separator used either in “vacuum” or “aspiration” mode.
Discussion
Spurious hemolysis is a critical challenge in laboratory diagnostics, since it consistently decreases sample quality and may virtually jeopardize patient safety when unreliable results produced on hemolyzed specimens are released to the stakeholders (11-14). Blood drawing is indeed the prevailing cause of in vitro hemolysis, which may be further worsened by the increased and prolonged fluid shear stress on blood caused by the vacuum of primary blood collection tubes, thus ultimately enhancing the risk of erythrocyte injury (18). As such, the use of disposals that mitigate the physical stress of blood while preserving operator safety are strongly advisable (11). Although the S-Monovette tube system represents an appealing perspective since it combines “vacuum” and “aspiration” mode, no previous studies have assessed its effectiveness in reducing the chance of erythrocytes injury and micro- or macro-hemolysis.
The results of this investigation attest that although the serum concentration of cell-free hemoglobin was always below the analytically and clinically significant threshold of 0.5 g/L in all samples (13,15), in serum specimens drawn by S-Monovette and aspiration mode the concentrations of potassium and LD were significantly lower than in those collected by vacuum with either tube S-Monovette or an analogous commercial evacuated tube system. Regardless of tube manufacturer, the vacuum aspiration also imposes an increased bias as compared with the aspiration mode, since the specification for allowable bias derived from biological variation (i.e., ± 1.8%) is exceeded for potassium. The specific composition of the S-Monovette tube per se cannot be considered an explanation for this finding, inasmuch as the values of both parameters did not significantly differ when blood was collected by vacuum with either BD Vacutainer or S-Monovette tubes. Neither the performance of two separate venipunctures can explain the observed bias, since it has been previously shown that when a standardized technique and identical disposals are used for collecting blood specimens during separate and sequential phlebotomies on the same patient, the bias is completely attributed to the single variable that has been modified between the first venipuncture and the following (19). Therefore, and in agreement with previous data (20), the occurrence of micro-hemolysis in blood drawn by evacuated tube systems is the most plausible explanation for our findings. These results were also confirmed in another independent study, in which Halm and Gleaves concluded that the burden of hemolyzed samples obtained with evacuated tube systems was nearly twice as high as that occurring with a syringe (i.e., 77% versus 49%) (21).
At variance with previous studies comparing aspiration and vacuum modes of blood collection, we have first showed that the use of S-Monovette - a system that reproduces the slow and gentle syringe aspiration - may be effective for reducing the burden of micro-hemolysis in serum blood specimens. The S-Monovette has however additional economic and safety advantages over traditional syringes, since it abates the incremental costs of the syringe itself, while substantially reducing the risk of needle-stick injury due to transfer of blood from the syringe to the tube. It is also noteworthy that the well known likelihood of erythrocyte injury and consequent spurious hemolysis caused by forceful transfer of blood from syringe to the tube (22) would also be virtually eliminated using S-Monovette in the aspiration mode.
Conclusion
We thereby conclude that in all conditions where it is predictable that a difficult venipuncture combined with the vacuum force of the primary tube may increase the probability of obtaining hemolyzed specimens, the S-Monovette system used in the aspiration mode represent an advantageous alternative to standard evacuated tube systems and traditional syringes.
Notes
Potential conflict of interest
None declared.
References
1. Lippi G, Simundic AM, Mattiuzzi C. Overview on patient safety in healthcare and laboratory diagnostics. Biochem Med 2010;20:131-43.
2. Lippi G, Guidi GC, Mattiuzzi C, Plebani M. Preanalytical variability: the dark side of the moon in laboratory testing. Clin Chem Lab Med 2006;44:358-65.
http://dx.doi.org/10.1515/CCLM.2006.073.
3. Lippi G, Chance JJ, Church S, Dazzi P, Fontana R, Giavarina D, et al. Preanalytical quality improvement: from dream to reality. Clin Chem Lab Med 2011;49:1113-26.
http://dx.doi.org/10.1515/cclm.2011.600.
4. Simundic AM, Lippi G. Preanalytical phase--a continuous challenge for laboratory professionals. Biochem Med 2012;22:145-9.
5. Lippi G, Salvagno GL, Montagnana M, Lima-Oliveira G, Guidi GC, Favaloro EJ. Quality standards for sample collection in coagulation testing. Semin Thromb Hemost 2012;38:565-75.
http://dx.doi.org/10.1055/s-0032-1315961.
6. Lippi G, Avanzini P, Cosmai M, Aloe R, Ernst D. Incomplete filling of lithium heparin tubes affects the activity of creatine kinase and gamma-glutamyltransferase. Br J Biomed Sci 2012;69:67-70.
7. Lima-Oliveira G, Lippi G, Salvagno GL, Montagnana M, Picheth G, Guidi GC. Preanalytical management: serum vacuum tubes validation for routine clinical chemistry. Biochem Med 2012;22:180-6.
8. Daves M, Lippi G, Cosio G, Raffagnini A, Peer E, Dangella A, et al. An unusual case of a primary blood collection tube with floating separator gel. J Clin Lab Anal 2012;26:246-7.
http://dx.doi.org/10.1002/jcla.21512.
10. Cuhadar S, Atay A, Koseoglu M, Dirican A, Hur A. Stability studies of common biochemical analytes in serum separator tubes with or without gel barrier subjected to various storage conditions. Biochem Med 2012;22:202-14.
11. Simundic AM, Topic E, Nikolac N, Lippi G. Hemolysis detection and management of hemolysed specimens. Biochem Med 2010;20:154-9.
12. Lippi G, Plebani M. Continuous-Flow Automation and Hemolysis Index: A Crucial Combination. J Lab Autom 2012 Jun 19. [Epub ahead of print].
14. Lippi G, Avanzini P, Pavesi F, Bardi M, Ippolito L, Aloe R, Favaloro EJ. Studies on in vitro hemolysis and utility of corrective formulas for reporting results on hemolyzed specimens. Biochem Med 2011;21:297-305.
15. Lippi G, Blanckaert N, Bonini P, Green S, Kitchen S, Palicka V, et al. Haemolysis: an overview of the leading cause of unsuitable specimens in clinical laboratories. Clin Chem Lab Med 2008;46:764-72.
http://dx.doi.org/10.1515/CCLM.2008.170.
16. Lippi G, Luca Salvagno G, Blanckaert N, Giavarina D, Green S, Kitchen S, et al. Multicenter evaluation of the hemolysis index in automated clinical chemistry systems. Clin Chem Lab Med 2009;47:934-9.
http://dx.doi.org/10.1515/CCLM.2009.218.
17. Ricos C, Alvarez V, Cava F, Garcia-Lario JV, Hernandez A, Jimenez CV, et al. Current databases on biologic variation: pros, cons and progress. Scand J Clin Lab Invest 1999;59:491-500.
http://dx.doi.org/10.1080/00365519950185229.
19. Lippi G, Salvagno GL, Brocco G, Guidi GC. Preanalytical variability in laboratory testing: influence of the blood drawing technique. Clin Chem Lab Med 2005;43:319-25.
http://dx.doi.org/10.1515/CCLM.2005.055.
20. Tamechika Y, Iwatani Y, Tohyama K, Ichihara K. Insufficient filling of vacuum tubes as a cause of microhemolysis and elevated serum lactate dehydrogenase levels. Use of a data-mining technique in evaluation of questionable laboratory test results. Clin Chem Lab Med 2006;44:657-61.
http://dx.doi.org/10.1515/CCLM.2006.109.