Critical review and meta-analysis of spurious hemolysis in blood samples collected from intravenous catheters
Giuseppe Lippi
[*]
[1]
Gianfranco Cervellin
[2]
Camilla Mattiuzzi
[3]
Introduction
Preanalytical phase components are the leading causes of poor sample quality, wherein inappropriate or mishandled procedures for collecting blood specimens may be associated with a magnified risk of unsuitable samples (1,2). Among various preanalytical non-conformances that can be encountered in routine laboratory practice, sample hemolysis represents the primary source of problems, in terms of prevalence and likelihood of sample rejection (3,4). Spurious hemolysis, also referred to as “in vitro hemolysis”, is conventionally defined as erythrocyte injury or breakdown occurring during or after sample collection, once potential sources of hemolytic anemia have been ruled out. Although the observed frequency of spuriously hemolyzed samples varies widely throughout different healthcare settings, it can be estimated around ~3% of all serum or plasma samples referred to central laboratories for routine or stat testing (5). It is also noteworthy that the vast majority of hemolyzed specimens come from the emergency department (ED), where the relative prevalence can be as high as 8-12% (6). The most frequent causes of spurious hemolysis include troublesome venipuncture(s), use of inappropriate blood collection devices, inappropriate handling (i.e., vigorous mixing) and transportation (i.e., freezing or trauma) of blood tubes (4,7). Regardless of specific causes, the receipt of hemolyzed specimens is always a problem, wherein test results of some analytes such as potassium, lactate dehydrogenase (LD), aspartate aminotransferase (AST) or cardiospecific troponins among others should be suppressed, reported with comments, corrected or recalculated or even provided with semi-quantative comments indicating likely range of results (8,9). This obviously causes diagnostic delays, incremental costs for material used for redrawing blood, as well as interrelational problems with hospital physicians and nurses (6).
Among potential sources of erythrocyte injury, blood drawing through intravenous catheters has been reported as an important factor in a variety of studies and reviews, which however provided rather different estimations of frequency, nor have these studies exactly quantified the risk of catheter-related hemolysis (10,11). In this study we performed a systematic search of current scientific literature, to estimate the cumulative risk of spurious hemolysis in samples collected from intravenous catheters.
Materials and methods
Search methodology
A systematic electronic search was performed on the three most frequently used scientific databases (i.e., PubMed, Web of Science and Scopus) (12), with no date restriction, to retrieve all published studies up to February 2013 that investigated the rate of hemolysis in blood drawn by intravenous catheters, either comparing intravenous catheter collection combined with evacuated blood tubes versus straight needle collection combined with evacuated blood tubes, or intravenous catheter collection combined with evacuated blood tubes versus intravenous catheter collection combined with manual aspiration of blood. The following keywords were used: “hemolysis” or “haemolysis”, in combination with “catheter” or “intravenous line”. The bibliographic references of items published in English, French, Spanish and Italian were reviewed for additional relevant studies. All the articles identified according to these search criteria were systematically assessed for quality by two authors (GL and CM), according to the Quality Assessment of Diagnostic Accuracy Studies (QUADAS) checklist criteria (13). Disagreements were solved by a third opinion (GC).
Statistical analysis
Heterogeneity was assessed by chi-square based statistics and I-square test, whereas the publication bias was evaluated using Egger test. A sensitivity analysis was performed according to publication as full-length article. The Odds ratio (OR), Relative risk (RR) and 95% Confidence interval (95% CI) were calculated using a random effect model, as for I-square values greater than 50%. Statistical analysis was performed with MedCalc Version 12.3.0 (MedCalc Software, Mariakerke, Belgium).
Results
The electronic search according to the above mentioned criteria identified 117 citations of articles and abstracts after elimination of replicates among the searchable databases (Figure 1). Sixty seven items were immediately excluded after title and/or abstract consultation, because not compliant with the aim of this study (i.e., most of these were microbiological studies assessing the hemolysis potential of bacteria and other microorganisms). Eighteen items were excluded after full text reading because not showing original data (i.e., editorials or critical reviews of the literature), whereas 15 articles were excluded because they did not contain specific data about hemolysis. The remaining 17 items were carefully assessed for quality after revision of the text, and two were excluded because they did not contain sufficient information for calculating OR and RR. Inter-rater agreement was excellent (98%; kappa statistic = 0.88; P < 0.001).
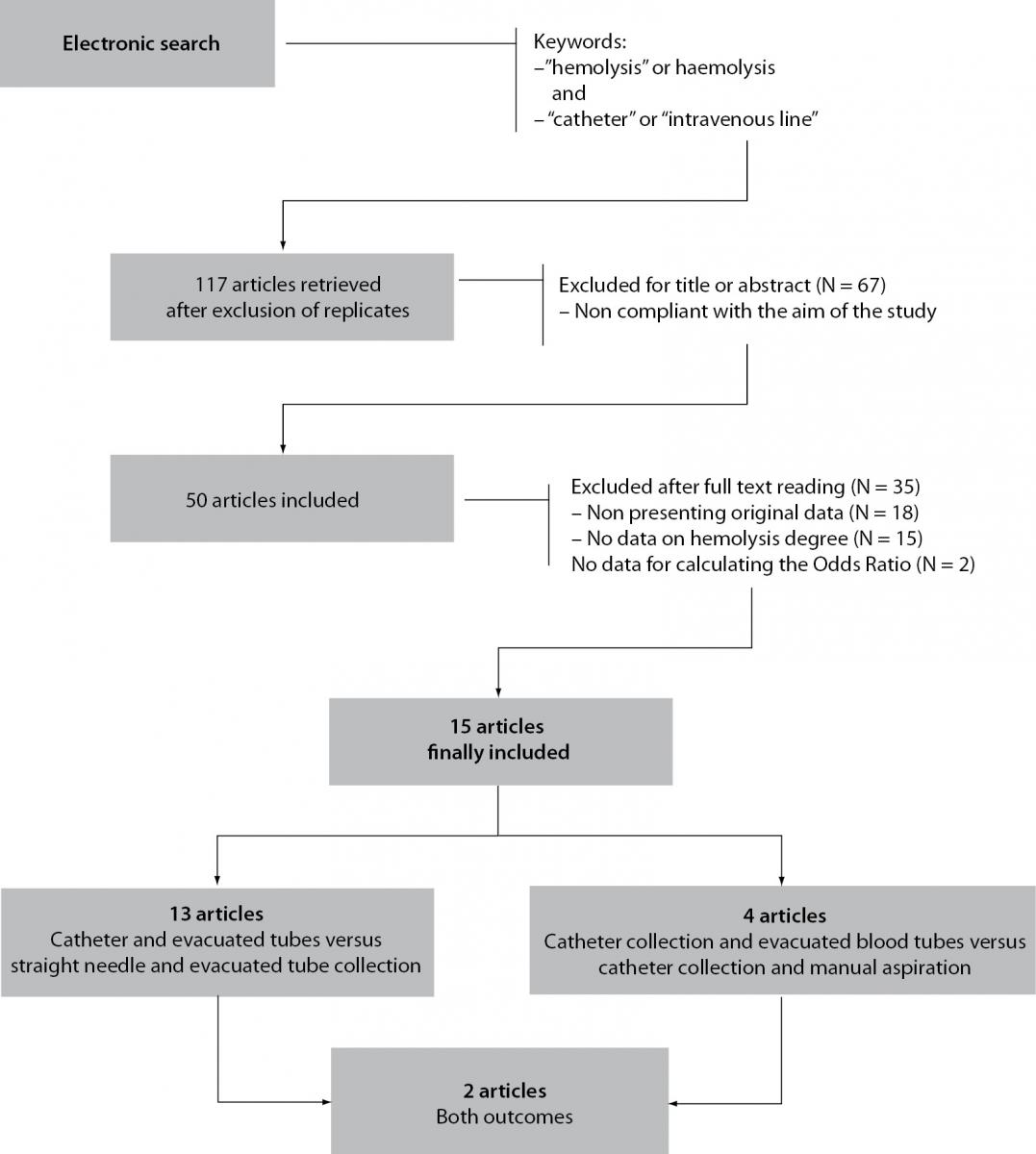
Figure 1. Prospect of electronic search and study evaluation.
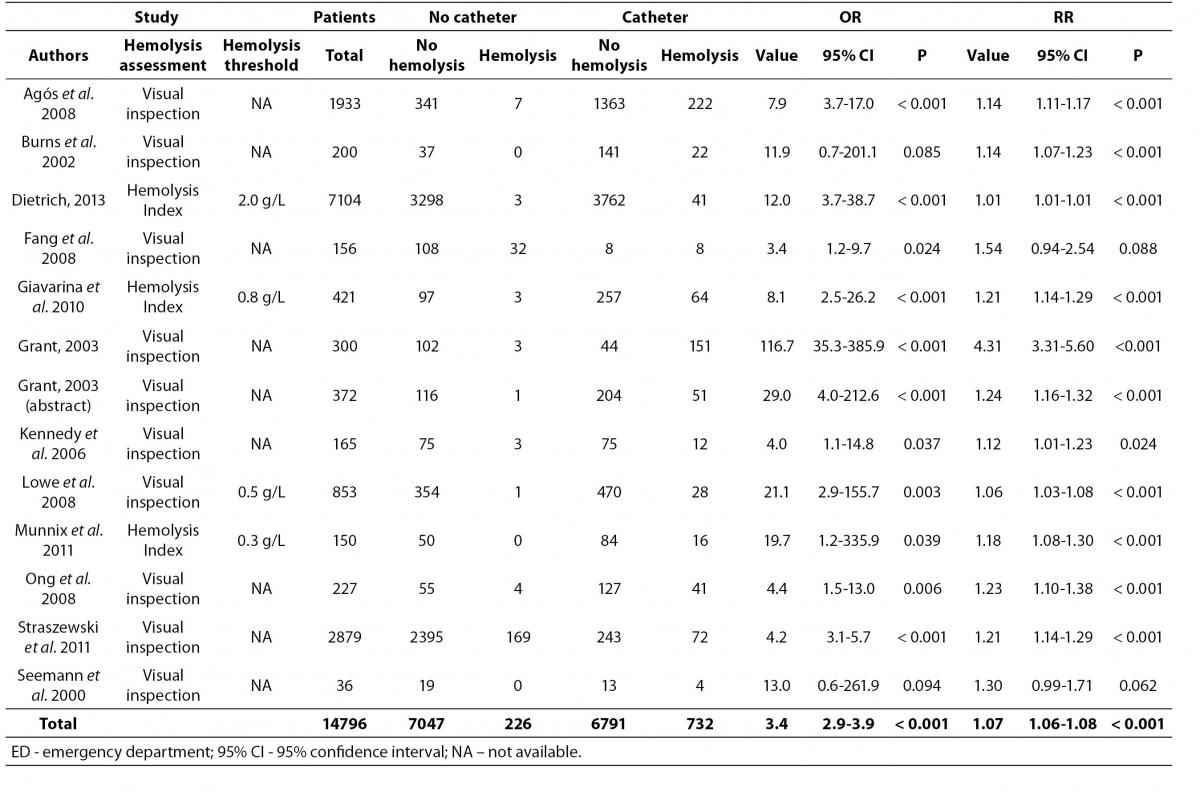
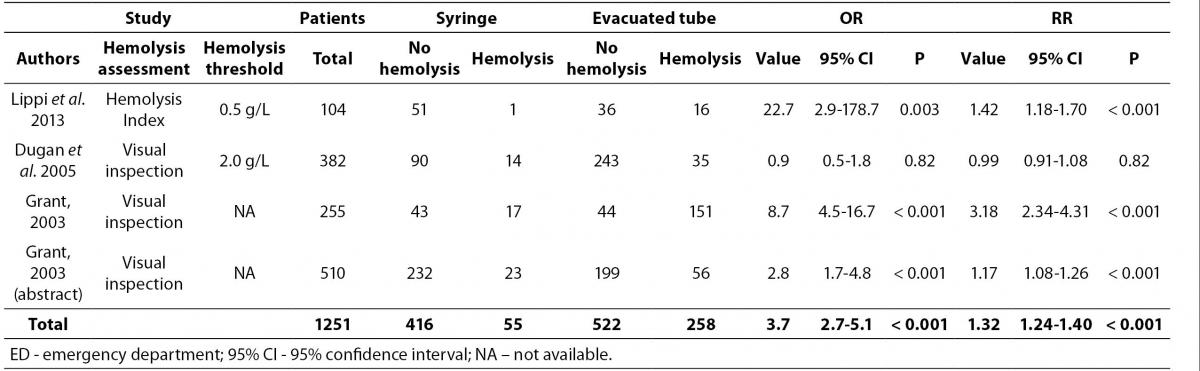
Overall, 15 articles were finally selected for inclusion in meta-analysis (14-28). Eleven of these articles showed data on catheter combined with evacuated tubes collection versus straight needle combined with evacuated tubes collection, two on catheter combined with evacuated blood tubes collection versus catheter combined with manual aspiration of blood, and two on both study outcomes (Figure 1). Therefore, the final number of studies assessing catheter combined with evacuated tubes collection versus straight needle combined with evacuated tubes collection was 13, whereas those comparing catheter combined with evacuated blood tubes collection versus catheter combined with manual aspiration were 4, for a total of 17 studies in the 15 articles included. The total number of patients was 14,796 in 13 studies assessing catheter combined with evacuated tubes versus straight needle combined with evacuated tubes, and 1251 in 4 studies assessing catheter combined with evacuated tubes versus catheter combined with manual blood aspiration. The inter-study variation was high and attributable to heterogeneity for sample size (chi-squared, 3185; DF, 17; I-squared, 99.8%; P < 0.001), modality of sample hemolysis assessment (chi-squared, 1699; DF, 17; I-squared, 99.0%; P < 0.001), as well as for hemolysis threshold (chi-squared, 1172; DF, 17; I-squared, 98.6%; P < 0.001). All studies were based in EDs. No publication bias was found by Egger regression test (P = 0.68).
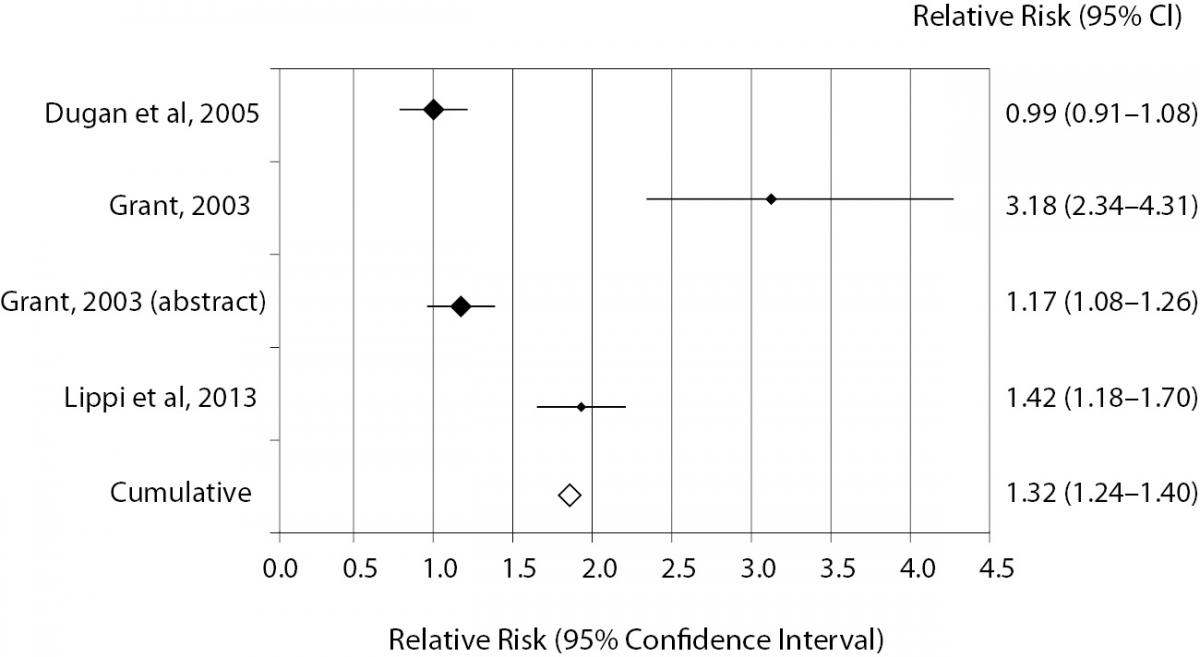
The results of individual studies along with random effect ORs and RRs for catheter-related hemolysis are shown in table 1 and 2. The range of individual ORs (3.4-116.7) and RRs (1.01-4.31) were statistically significant in 11/13 studies comparing catheter and evacuated tubes with straight needle and evacuated tubes, whereas the range of individual ORs (0.9-22.7) and RRs (0.99-3.18) were statistically significant in 3/4 studies comparing catheter and evacuated tubes with catheter and manual blood aspiration. Significant risk of spurious hemolysis was found pooling results of 13 individual studies assessing catheter and evacuated tubes versus straight needle and evacuated tubes, showing random effect OR of 3.4 (95% CI = 2.9-3.9; P < 0.001) and RR of 1.07 (95% CI = 1.06-1.08; P < 0.001) (Table 1). The sensitivity analysis did not reveal a significant impact of publication status on these results after excluding trials that were not published as full-length articles (OR 3.1 versus 3.4; RR 1.07 for both cases). Similarly, a statistically significant risk was observed pooling results of 4 individual studies assessing catheter and evacuated tubes versus catheter and manual blood aspiration blood, showing random effect OR of 3.7 (95% CI = 2.7-5.1; P < 0.001) and RR of 1.32 (95% CI = 1.24-1.40; P < 0.001) (Table 2 and Figure 2). The sensitivity analysis did not reveal a significant impact of publication status on these results after excluding the trial that was not published as full-length article (OR 3.6 versus 3.7; RR 1.40 versus1.32). No study directly compared catheter and manual aspiration collection versus straight needle and evacuated tube.
Table 1. Individual studies and random effect Odds ratio (OR) and Relative risk (RR) of spurious hemolysis in catheter combined with evacuated tubes collection versus straight needle combined with evacuated tube collection in the emergency department.
Table 2. Individual studies and random effect Odds ratio (OR) and Relative risk (RR) of spurious hemolysis in catheter combined with evacuated tubes collection versus catheter combined with manual aspiration collection in the emergency department.
Figure 2. Individual studies and random effect Relative risk (RR) of spurious hemolysis in catheter combined with evacuated tubes collection versus catheter combined with manual aspiration collection.
Discussion
The results of this meta-analysis - which is limited to published data and ED setting - attest that sample collection through intravenous catheters is associated with significantly higher risk (i.e., 7%) of spurious hemolysis as compared with standard blood drawn by straight needles, and that this risk is further amplified when intravenous catheter are associated with primary evacuated blood tubes as compared with manual blood aspiration with syringes or S-Monovette® blood tubes (Sarstedt AG & Co., Nümbrecht, Germany) used in manual aspiration mode (29). According to these findings, it seems reasonable to conclude that blood drawing by straight needle venipuncture should be preferred over intravenous catheter collection for reducing the chance of erythrocyte injury, although this approach may appear impractical in healthcare setting such as ED or Intensive care units (ICU) where intravenous lines are systematically placed upon patient admission. A potential option to reduce the chance of collecting unsuitable samples entails the use of manual aspiration rather than vacuum force for drawing blood from intravenous catheters (Figure 2), since the former practice causes a larger shear stress due to the collapse of soft plastic material under negative pressure when blood is aspirated by the vacuum, as well as turbulence due to difference of pressures between veins, catheter needles, valves and evacuated collection tubes (11).
The large heterogeneity in methods used to identify unsuitable samples (i.e., hemolysis index versus visual inspection), which is partially attributable to the fact that automatic performance of serum indices has only recently become available in laboratory instrumentation (30), is another important finding, which calls for additional practical considerations. First, visual inspection is arbitrary, cannot be standardized and is characterized by small inter-observer agreement (31), so that it should be replaced by automated systems that report serum indices. The hemolysis index is in fact accurate when compared with the reference cyanmethemoglobin assay, and is also highly reproducible among different platforms and laboratories (31,32). This aspect becomes crucial considering that increasing implementation of continuous-flow automation in clinical laboratory abolishes the chance of visually inspecting the samples, thus making automatic performance of serum indices virtually unavoidable (31,33). Inherently linked to this point is the lack of consensus about hemolysis thresholds across studies where the cut-off of cell-free hemoglobin has been reported (ranging from 0.3 up to 2.0 g/L). Besides Italian national recommendations, concluding that a sample should be considered “modestly” or “frankly” hemolyzed when cell-free hemoglobin concentration is 0.3-0.6 g/L and > 0.6 g/L, respectively (34), there are no other guidelines about this issue. This is a gap that the new European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) working group on preanalytical variability is supposed to close, by issuing official recommendations for harmonizing policies for rejecting hemolyzed samples throughout Europe (35).
Notes
Potential conflict of interest
None declared.
References
1. Lippi G, Chance JJ, Church S, Dazzi P, Fontana R, Giavarina D, et al. Preanalytical quality improvement: from dream to reality. Clin Chem Lab Med 2011;49:1113-26.
http://dx.doi.org/10.1515/cclm.2011.600.
3. Lippi G, Salvagno GL, Montagnana M, Lima-Oliveira G, Guidi GC, Favaloro EJ. Quality standards for sample collection in coagulation testing. Semin Thromb Hemost 2012;38:565-75.
http://dx.doi.org/10.1055/s-0032-1315961.
5. Carraro P, Servidio G, Plebani M. Hemolyzed specimens: a reason for rejection or a clinical challenge? Clin Chem 2000;46:306-7.
7. Lippi G, Blanckaert N, Bonini P, Green S, Kitchen S, Palicka V, et al. Haemolysis: an overview of the leading cause of unsuitable specimens in clinical laboratories. Clin Chem Lab Med 2008;46:764-72.
http://dx.doi.org/10.1515/CCLM.2008.170.
8. Lippi G, Avanzini P, Pavesi F, Bardi M, Ippolito L, Aloe R, Favaloro EJ. Studies on in vitro hemolysis and utility of corrective formulas for reporting results on hemolyzed specimens. Biochem Med 2011;21:297-305.
http://dx.doi.org/10.11613/BM.2011.040.
9. Lippi G, Avanzini P, Dipalo M, Aloe R, Cervellin G. Influence of hemolysis on troponin testing: studies on Beckman Coulter UniCel Dxl 800 Accu-TnI and overview of the literature. Clin Chem Lab Med 2011;49:2097-100.
http://dx.doi.org/10.1515/CCLM.2011.703.
10. Heyer NJ, Derzon JH, Winges L, Shaw C, Mass D, Snyder SR, et al. Effectiveness of practices to reduce blood sample hemolysis in EDs: a laboratory medicine best practices systematic review and meta-analysis. Clin Biochem 2012;45:1012-32.
http://dx.doi.org/10.1016/j.clinbiochem.2012.08.002.
12. Lippi G, Favalor EJ, Simundic AM. Biomedical research platforms and their influence on article submissions and journal rankings: an update. Biochem Med 2012;22:7-14.
http://dx.doi.org/10.11613/BM.2012.002.
13. Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3:25.
http://dx.doi.org/10.1186/1471-2288-3-25.
14. Agós MD, Lizarraga R, Gambra D, Marañón A, Orozco C, Díaz E. Factors related to haemolysis in the extraction of blood samples. An Sist Sanit Navar 2008;31:153-8.
16. Dietrich H. One Poke or Two: Can Intravenous Catheters Provide an Acceptable Blood Sample? A Data Set Presentation, Review of Previous Data Sets, and Discussion. J Emerg Nurs 2013 [Epub ahead of print].
http://dx.doi.org/10.1016/j.jen.2012.11.002.
18. Giavarina D, Pasquale L, Mezzena G, Soffiati G. Hemolysis by peripheral intravenous catheters: materials comparison. RIMeL/IJLaM 2010;6:216-21.
21. Kennedy C, Angermuller S, King R, Noviello S, Walker J, Warden J, Vang S. A comparison of hemolysis rates using intravenous catheters versus venipuncture tubes for obtaining blood samples. J Emerg Nurs 1996;22:566-9.
http://dx.doi.org/10.1016/S0099-1767(96)80213-3.
22. Lowe G, Stike R, Pollack M, Bosley J, O’Brien P, Hake A, et al. Nursing blood specimen collection techniques and hemolysis rates in an emergency department: analysis of venipuncture versus intravenous catheter collection techniques. J Emerg Nurs 2008;34:26-32.
http://dx.doi.org/10.1016/j.jen.2007.02.006.
23. Munnix IC, Schellart M, Gorissen C, Kleinveld HA. Factors reducing hemolysis rates in blood samples from the emergency department. Clin Chem Lab Med 2011;49:157-8.
http://dx.doi.org/10.1515/cclm.2011.012.
24. Ong ME, Chan YH, Lim CS. Observational study to determine factors associated with blood sample haemolysis in the emergency department. Ann Acad Med Singapore 2008;37:745-8.
25. Straszewski SM, Sanchez L, McGillicuddy D, Boyd K, Dufresne J, Joyce N, et al. Use of separate venipunctures for IV access and laboratory studies decreases hemolysis rates. Intern Emerg Med 2011;6:357-9.
http://dx.doi.org/10.1007/s11739-011-0568-9.
26. Seemann S, Reinhardt A. Blood sample collection from a peripheral catheter system compared with phlebotomy. J Intraven Nurs 2000;23:290-7.
28. Dugan L, Leech L, Speroni KG, Corriher J. Factors affecting hemolysis rates in blood samples drawn from newly placed IV sites in the emergency department. J Emerg Nurs 2005;31:338-45.
http://dx.doi.org/10.1016/j.jen.2005.05.004.
29. Lippi G, Avanzini P, Musa R, Sandei F, Aloe R, Cervellin G. Evaluation of sample hemolysis in blood collected by S-Monovette® using vacuum or aspiration mode. Biochem Med 2013;23:64-9.
http://dx.doi.org/10.11613/BM.2013.008.
31. Simundic AM, Nikolac N, Ivankovic V, Ferenec-Ruzic D, Magdic B, Kvaternik M, Topic E. Comparison of visual vs. automated detection of lipemic, icteric and hemolyzed specimens: can we rely on a human eye? Clin Chem Lab Med 2009;47:1361-5.
http://dx.doi.org/10.1515/CCLM.2009.306.
32. Lippi G, Luca Salvagno G, Blanckaert N, Giavarina D, Green S, Kitchen S, et al. Multicenter evaluation of the hemolysis index in automated clinical chemistry systems. Clin Chem Lab Med 2009;47:934-9.
http://dx.doi.org/10.1515/CCLM.2009.218.
33. Lippi G, Plebani M. Continuous-Flow Automation and Hemolysis Index: A Crucial Combination. J Lab Autom 2012 [Epub ahead of print].
34. Lippi G, Caputo M, Banfi G, Daves M, Dolci A, Montagnana M, et al. SIBioC-SIMeL consensus recommendations for the identification and management of hemolysed specimens and the implementation of hemolysis index. Biochim Clin 2011;35:481-90.