Effect of sample type, centrifugation and storage conditions on vitamin D concentration
Ayfer Colak
[1]
Burak Toprak
[*]
[2]
Nese Dogan
[2]
Fusun Ustuner
[2]
Introduction
25-hydroxyvitamin D [25(OH)D] is the most important vitamin D form in the circulation and the most reliable indicator of vitamin D storage (1). The half-life of 25(OH)D is approximately 20 days and therefore it is considered as the parameter that best reflects the vitamin D condition (synthesis, uptake and consumption) in the organism. Vitamin D is found mostly as 25(OH)D3 in the blood, which is the main form of storage (2).
Older studies about long-term stability of vitamin D reported that samples stored at -20 °C showed 10% decrease in vitamin D concentration after 3 months (3,4). In addition, after evaluating short-term vitamin D stability in serum and plasma samples, one older study reported absence of significant change at 24 °C during 72 hours (5). However, these studies have some limitations such as small sample size and imprecision of the assay used. Stability studies conducted in the last decade evaluated vitamin D metabolites only in serum in a limited number of samples (6-8). Multiple freeze thaw cycles and delayed centrifugation were reported not to affect vitamin D concentration (9), though measurement may be affected by some other preanalytical factors, like capillary sampling, prolonged direct sunlight exposure, and in some assays, the choice of collection tube (8,10,11).
Although vitamin D is considered as a relatively stable analyte, effect of preanalytical conditions and stability of vitamin D in serum and plasma needs to be identified clearly due to the limitations of previous studies. Also, as vitamin D is a test not determined in every laboratory and samples are very often shipped for analyte determination, it has been considered that optimization of storage and transfer conditions is necessary to minimize pre-analytical errors.
Therefore, objectives of this study were: i) to investigate the difference between serum and plasma, as well as the effect of centrifugation procedures and the effect of standing conditions in normal routine run on the vitamin D concentration measurement; ii) to evaluate the storage conditions for samples not to be run on the same day and to be shipped to an external laboratory.
Materials and methods
Subjects
The study was performed in Tepecik Training and Research Hospital Biochemistry Department, Izmir, Turkey, between February and May 2012. Fifteen voluntary laboratory employees aged 27-63 years were enrolled in the study. Subjects were healthy adults, with no known diagnosis of cancer, diabetes mellitus, cardiovascular diseases and osteoporosis. Information about the study was provided for all participants. Participants have completed an informed consent form. The study was approved by the Tepecik Training and Research Hospital Ethics Committee and complies with the ethical principles of the Declaration of Helsinki.
Methods
Sample collection was completed within two days. Each participant was present at laboratory in the morning of sampling day, after fasting for 8-12 hours without taking any medicine. Each participant rested in a seated position prior sampling. Blood sampling was performed by one experienced laboratory technician.
Venous blood was collected into two 8.5 mL serum separator tubes (BD Vacutainer; Becton Dickinson, Meylan, France) with clot activator and three 2 mL EDTA tubes (BD Vacutainer; Becton Dickinson, Meylan, France). Samples were divided into two sets: low temperature conditions and normal laboratory conditions (Figure 1). At low temperature conditions, EDTA samples were centrifuged in refrigerated centrifuge (2-8 °C) immediately after blood collection. Serum samples were placed at 2-8 °C for 30 min to allow proper clot formation, and then centrifuged in refrigerated centrifuge. At normal laboratory conditions, all samples were centrifuged at room temperature: EDTA samples immediately and serum tubes after 30 minutes in upright position. All collection tubes were centrifuged at 3000 x g for 10 minutes. Immediately after centrifugation, both serum and EDTA samples of normal laboratory conditions were aliquoted into 3 vials. Time intervals and storage conditions for aliquots of normal laboratory conditions were as follows: 1) one vial for zero time measurement, 2) 4 hours at room temperature, and 3) 24 hours at 2-8 °C.
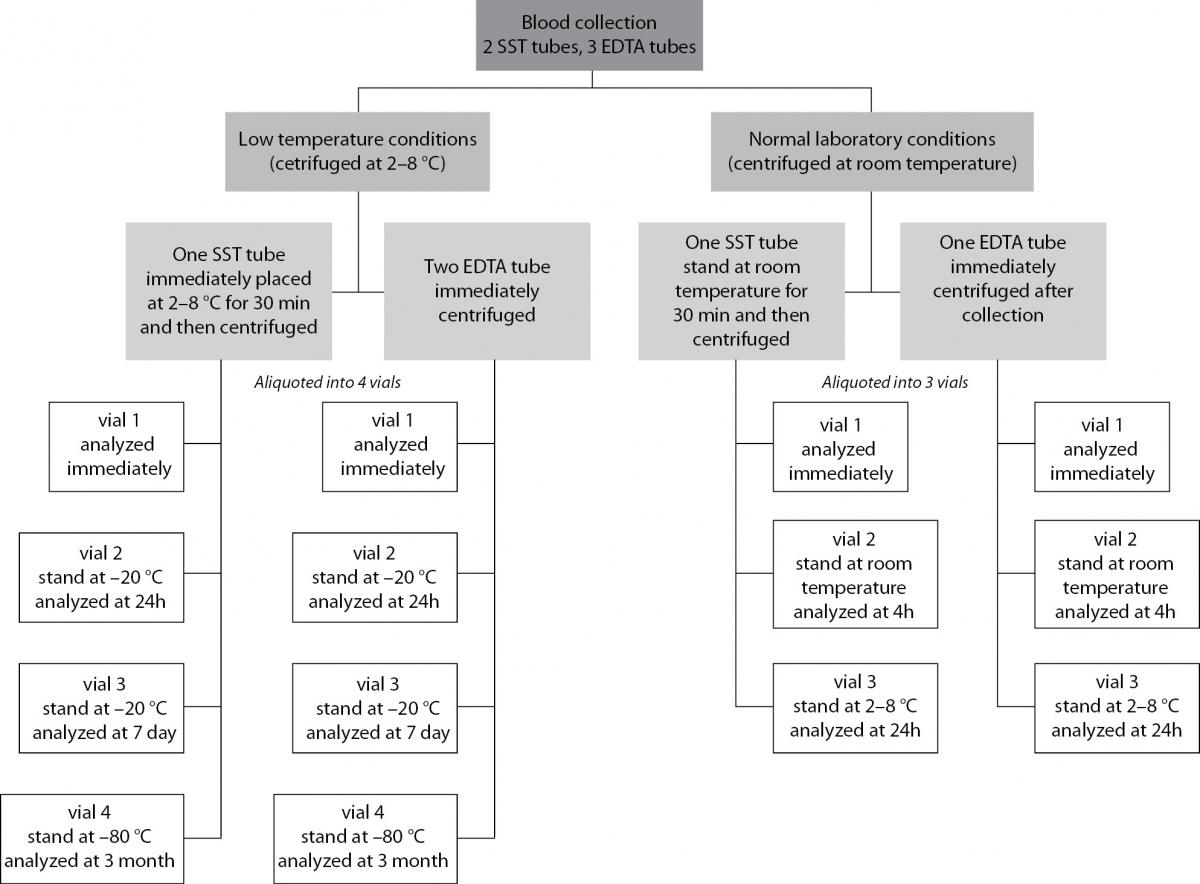
Figure 1. Flowchart of the study design.
Both serum and EDTA samples of low temperature conditions were aliquoted into 4 vials. Time intervals and storage temperatures for aliquots of low laboratory conditions were as follows: 1) one vial for zero time measurement, 2) 24 hours at -20 °C, 3) days at -20 °C, and 4) 3 months at -80 °C.
25(OH)D concentration was measured on Cobas E 411 immunoanalyzer (Roche Diagnostics, Mannheim, Germany). Prior to determination of vitamin D concentration for each study period, device was calibrated with original calibrator and verified with internal quality control serum (Cobas PreciControl Varia). The within-run and within-laboratory coefficients of variation were 5.1% and 8.5% at 37.5 nmol/L, 3.1% and 5.2% at 70 nmol/L.
Statistical analysis
Data analysis was done using SPSS version 15 (SPSS Inc., Chicago, IL, USA). Normality of distribution was tested using the Kolmogorov-Smirnov test. As the data did not follow normal distribution (P = 0.036 for plasma, P = 0.046 for serum), Wilcoxon signed rank test and Friedman repeated measures ANOVA were used for comparison of dependent samples. Results were presented as median and interquartile range. P values ≤ 0.05 were considered statistically significant. Explanatory variables included centrifugation method (two categories), collection tube (two categories), and storage time. In our study, all comparisons were made against zero measurements(plasma, serum, normal centrifuge, refrigerated centrifuge).
Results
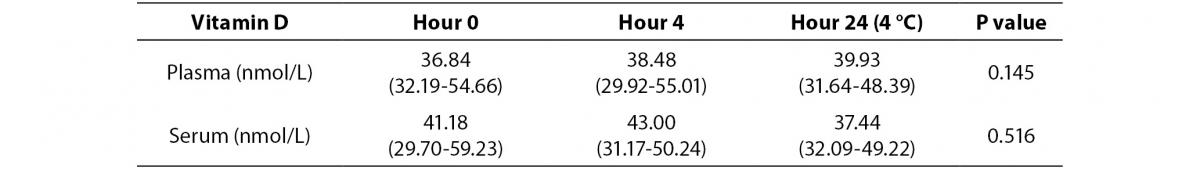
Plasma and serum 25(OH)D concentrations for samples subjected to normal and low temperature at different time intervals are shown in table 1 and table 2. In normal temperature conditions, plasma concentration of vitamin D did not differ from serum concentration at zero time (P = 0.088). There was no statistically significant difference between zero time, storage for 4 hours at room temperature and 24 hours at 2-8 °C (Friedman-ANOVA, P = 0.145 for plasma, P = 0.516 for serum).
Table 1. Median and interquartile range values of hour 0, hour 4 (room temperature) and hour 24 (4 °C) results after centrifugation of plasma and serum samples at room temperature.
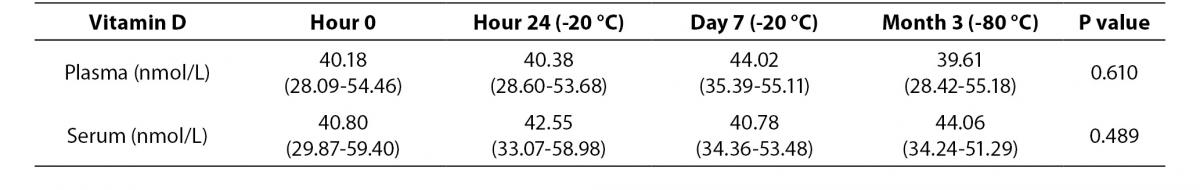
Table 2. Median and interquartile range values of hour 0, hour 24, day 7 and month 3 results of plasma and serum samples at low temperature conditions.
In low temperature conditions, plasma concentrations of vitamin D were not significantly different from serum vitamin D concentration at zero time (P = 0.061). There was no statistically significant difference in vitamin D concentration between zero time, storage for 24 hour at -20 °C, for 7 days at -20 °C and for 3 months at -80 °C (Friedman-ANOVA, P = 0.610 for plasma, P = 0.489 for serum).
No significant difference was observed between normal centrifuge and refrigerated centrifuge for both plasma and serum samples at zero time (P = 0.464 and 0.731, respectively).
Discussion
In this study, we have investigated common storage conditions: 4 hours at room temperature, 24 hours at 2-8 °C and -20 °C, 7 days at -20 °C and 3 months at -80 °C. 25(OH)D concentration did not show significant differences during the investigated time and temperature intervals. We found vitamin D to be a stable analyte consistent with other studies (5-7).
This was the first study evaluating the effect of centrifugation temperature on vitamin D concentration. No differences were observed between refrigerated centrifuge (2-8 °C) and centrifuge at room temperature. Centrifugation delays were investigated by another study and no significant differences were reported up to 96 hour after blood collection (10). It seems that vitamin D requires no special attention in terms of centrifugation.
Current study investigated both serum and plasma samples and the results showed that sample type did not affect stability. Both serum and plasma can be used for storage. The difference in 25(OH)D concentrations according to tube type in some assays was reported by another study for heparinized plasma (10).
The main limitation of this study is the storage conditions investigated. Storage conditions are short time intervals but our objective was to examine possible conditions that could be encountered commonly.
In conclusion our data confirmed that vitamin D is stable under common storage conditions. There is no significant difference between serum and plasma vitamin D and centrifugation temperature has no effect on vitamin D concentration.
Acknowledgement
The authors are thankful to Roche Diagnostics for providing reagents for this study.