Introduction
Laboratory medicine is undergoing continuous changes, due to medical information improvement, novel analytical technologies development and introduction of new tests. In order to ensure the best possible laboratory service to the patient, it is essential for laboratory medicine specialists to keep abreast with a broad range of new-emerging issues that have the potential to influence laboratory practice and patient care. The most appropriate way to achieve this goal is integration of continuing professional development (CPD) in the clinical laboratory education continuum.
Although clinical laboratory professionals in Europe have heterogeneous academic background (i.e. medicine, pharmacy or science), their professional practice is comparable in most Europe countries (1,2). Consequently, the majority of them have common professional register-European Register of Specialists in Clinical Chemistry and Laboratory Medicine- (EC4 register) and title Specialists in Laboratory Medicine(3). The main prerequisites for initial registration in EC4 are nine to ten years of under- and post-graduate studies. However, for renewal of EC4 licence, which is valid for 5 years, two major requirements exist: continuity in laboratory practice and participation in a CPD programme (2).
Literature sources usually mention two closely related, although not completely interchangeable terms for continuing education of laboratory specialists: CPD and continuing medical education (CME). The term CPD refers to the means by which people maintain knowledge and skills related to their professional status. Regarding health care providers, it consists of any educational activity that helps to maintain, develop or increase knowledge, problem-solving and technical skills or professional performance standards, all with the goal of providing the best possible health care (4). CME covers all specific forms of
continuing education that helps those in the
medical field to maintain competence and learn about new and developing areas (5). The term CPD should be preferably used since it promotes positive changes in practice, team management, communication and research, in addition to educational benefits, primarily issued by CME (6). There are several general requirements for successful implementation of CPD. It should be both individual and collective responsibility of the profession (4), coordinated by national or international professional organization, competent to offer an optimally structured spectrum of CPD activities (5). Accreditation of CPD activities is strongly recommended and their quality, referring both to providers and attendees, should be independently, regularly and objectively assessed and monitored (6). Concerning individual evaluation, CPD crediting system has been proven as the most efficient way for evaluation of CPD activities. However, the approach to CPD implementation and accompanying crediting system results in many variations across the countries.
European Federation of Clinical Chemistry and Laboratory Medicine (EFLM), as the European professional leader, is one of the authorities responsible for CPD activities among European specialists in laboratory medicine. The EFLM activities in this field are principally governed by two objectives. First is to recognize and evaluate current CPD programmes based on National rules within member countries. This should serve as a base for the second one - harmonisation with CPD programme and crediting system of the European Union of Medical Specialist (UEMS) - section Laboratory Medicine (UEMS-SLM/MB), which represent the another European authority covering area of CPD in this profession. The achievement of these objectives will yield to the final goal - the standardisation of CPD crediting system, thus making it appropriate for all specialists in laboratory medicine in Europe.
The aim of this article is to present results of the survey on current CPD situation in EFLM member countries, with emphasis on crediting system.
Materials and methods
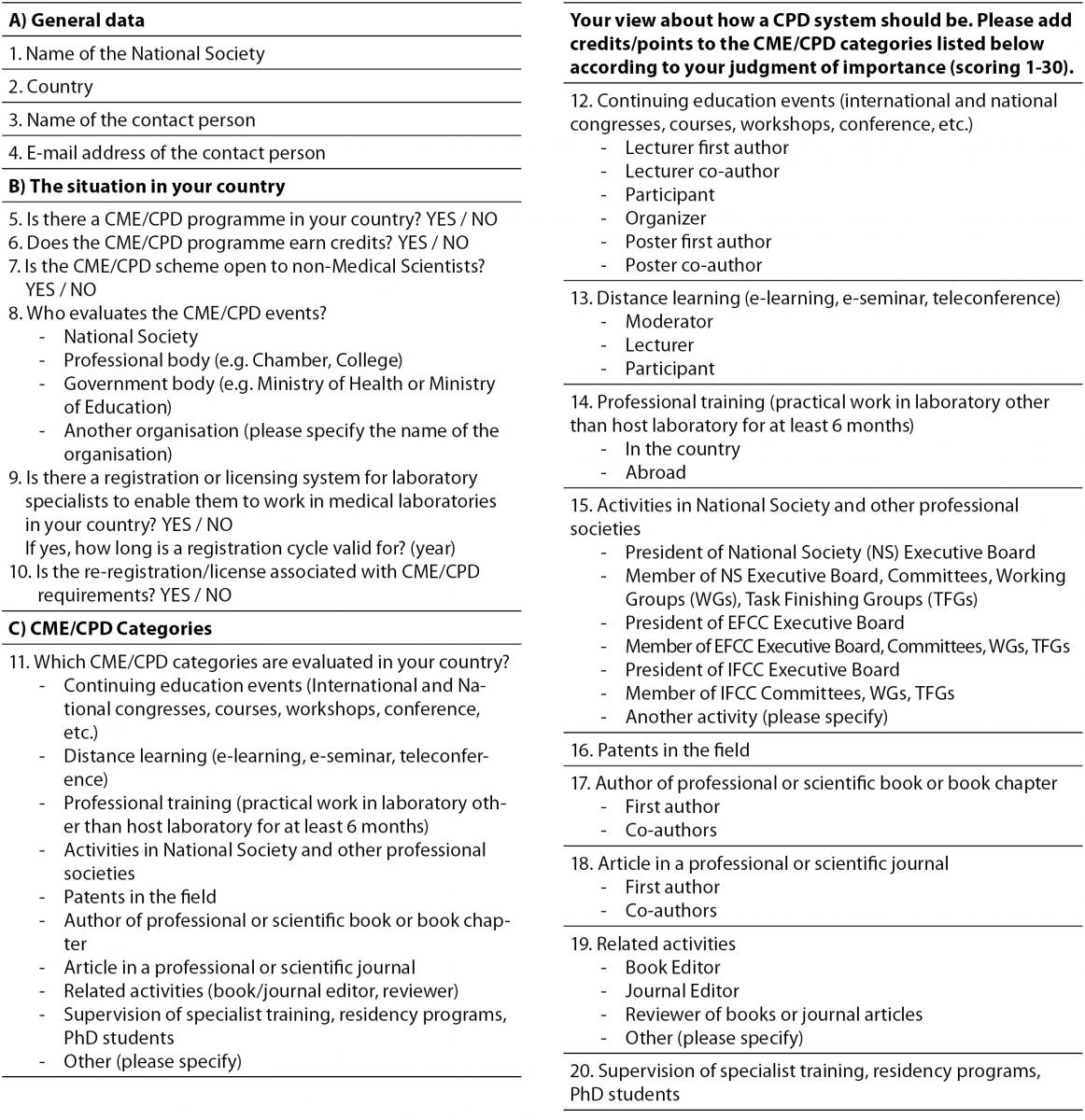
The survey questionnaire, shown in Table 1, consisted of 20 questions (using both CPD and CME terms) which were divided in three groups. In separate part of the third group, participants were offered nine different CME/CPD categories and asked to add 1-30 credits/points to each category according to their own judgment of importance. Terms CME and CPD were used interchangeably, because in some countries CME is usually used for medical and CPD for non-medical scientist working in medical laboratories. In discussion, only the term CPD will be used, because the article refers to CPD of laboratory professionals regardless of their basic educational background. The survey information along with an explanatory letter was forwarded to representatives of all 38 EFLM member societies at the end of 2011. Due to poor response in defined time the survey was re-sent in the first half of 2012. All data are expressed as ratios, absolute numbers, medians accompanied with interquartile range and modes. MedCalc® Statistical Software Vers. 12.7.2 (Frank Schoonjans, Mariakerke, Belgium) was used for calculation of descriptive statistic parameters.
Table 1. EFLM Survey: CPD system in Europe.
Results
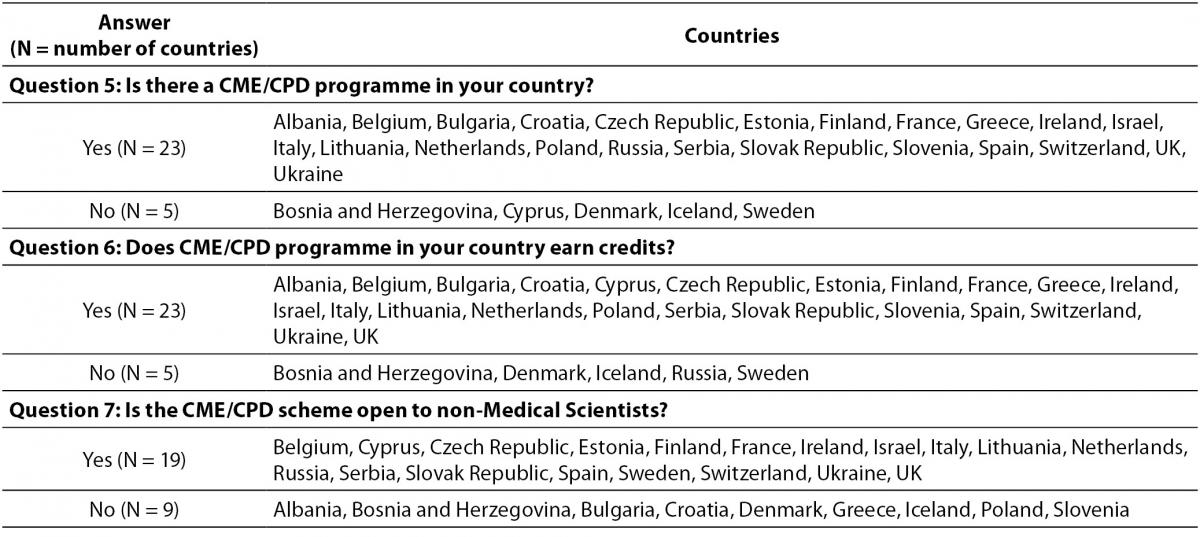
The countries that responded partially or fully (28/38) in the survey are shown in Table 2. Response to the questionnaire showed that 23/28 countries have a CPD programme, while five countries do not have any active programmes. The same ratio is observed for the countries where crediting system accompanies CPD. The CME/CPD programmes are open to non-medical scientists in 19/28 countries. Table 3 presents details about data collected for these questions.
Table 2. European countries which participated in the EFLM survey (N = 28).
Table 3. Answers on questions 5, 6 and 7.
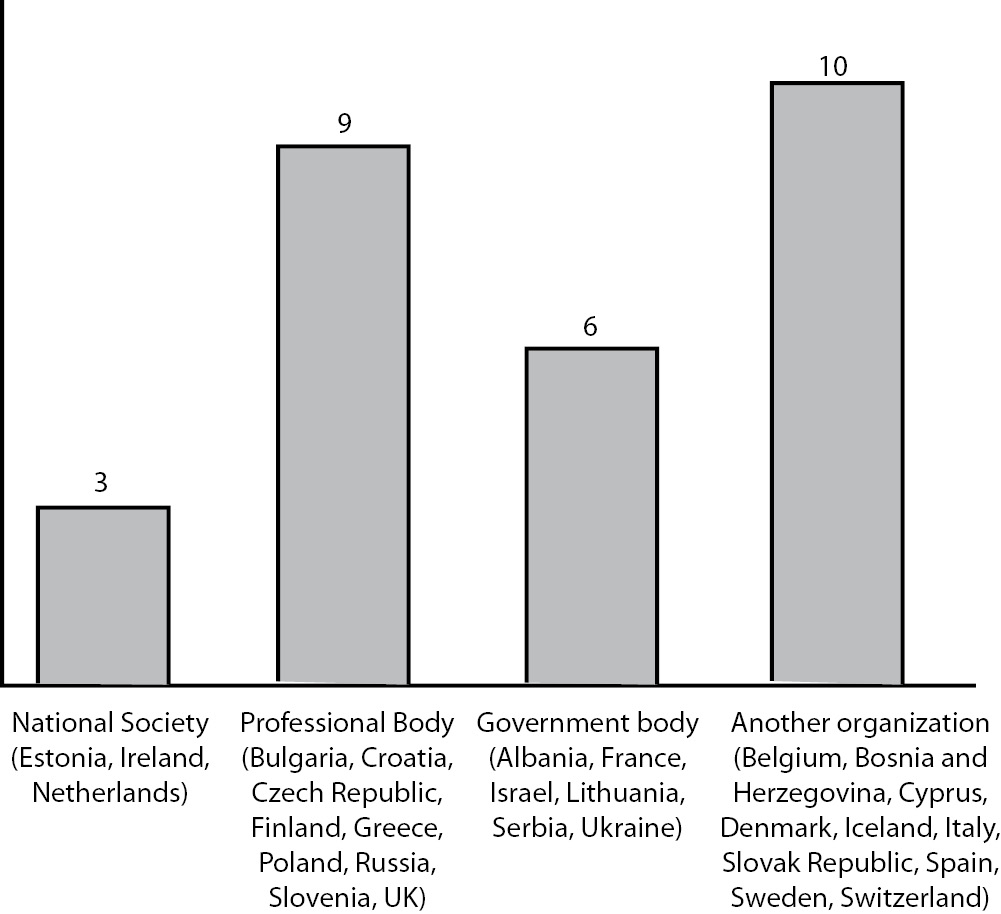
Regardless of the establishment of official CPD system, all participating countries reported that the CME/CPD events are evaluated. National societies were identified as the responsible authority in 3, professional and government bodies in 9 and 6 respectively, and other organisations in 10 participating countries (Figure1).
Figure 1. Who evaluates the CME/CPD events?
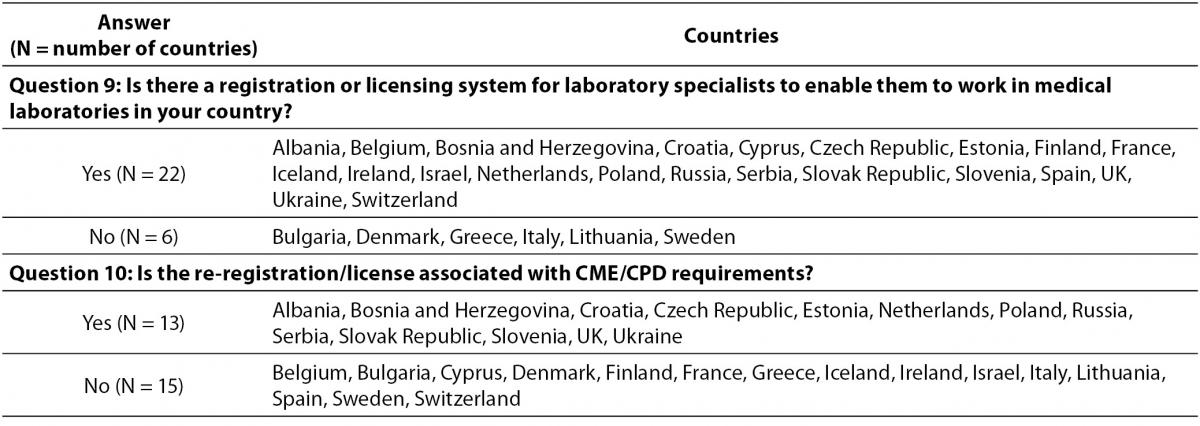
Registration or licensing system is implemented in 22/28 countries, with participation in CPD activities being a required for re-registration/licensing in 13 countries (Table 4). In Belgium, Cyprus, Finland, Iceland, Israel, Poland and Spain the validity of registration cycle has no limit. In other countries the validity time-span ranges from two (UK) until 10 years (Czech Republic), while four countries (Bosnia and Herzegovina, France, Ireland, and Italy) have no specifications on this issue.
Table 4. Relationship between participation in CPD activities in licensing and relicensing of specialists in laboratory medicine.
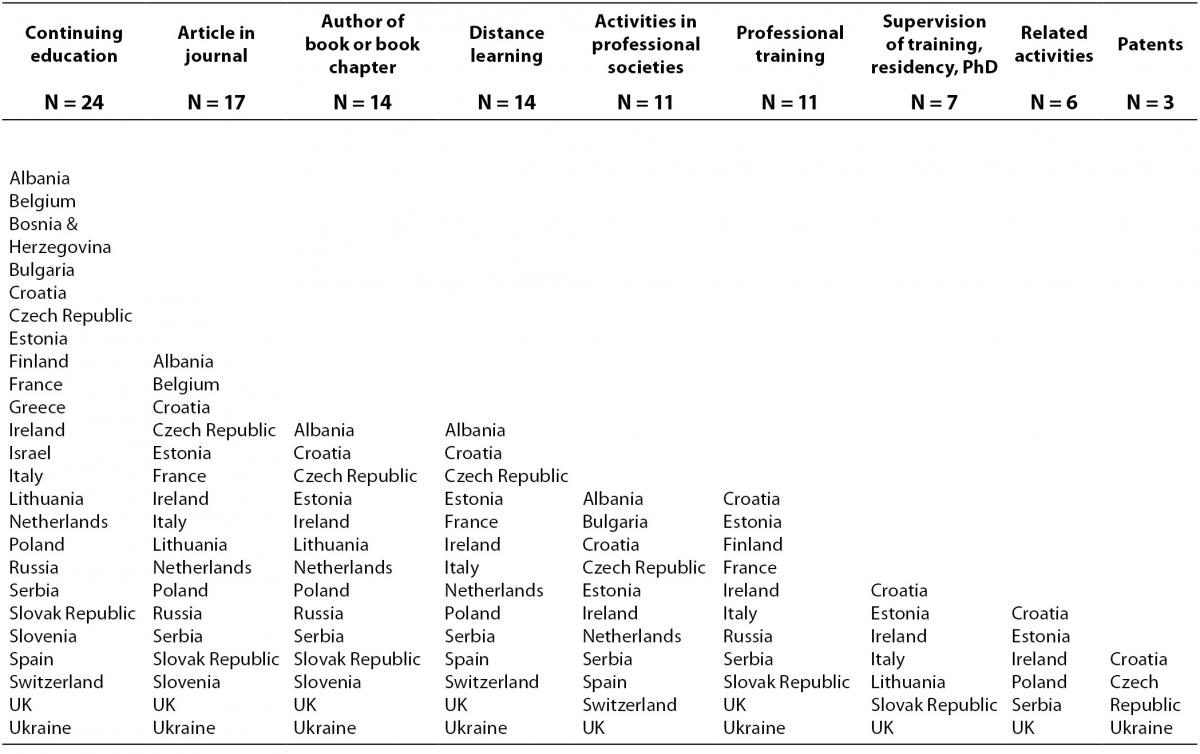
The data presented in Table 5, indicate the categories most often recognized as CPD activities: continuing education events, journal articles or book chapters, and distance learning.
Table 5. Categories recognised for CME/CPD in different countries (N = number of countries).
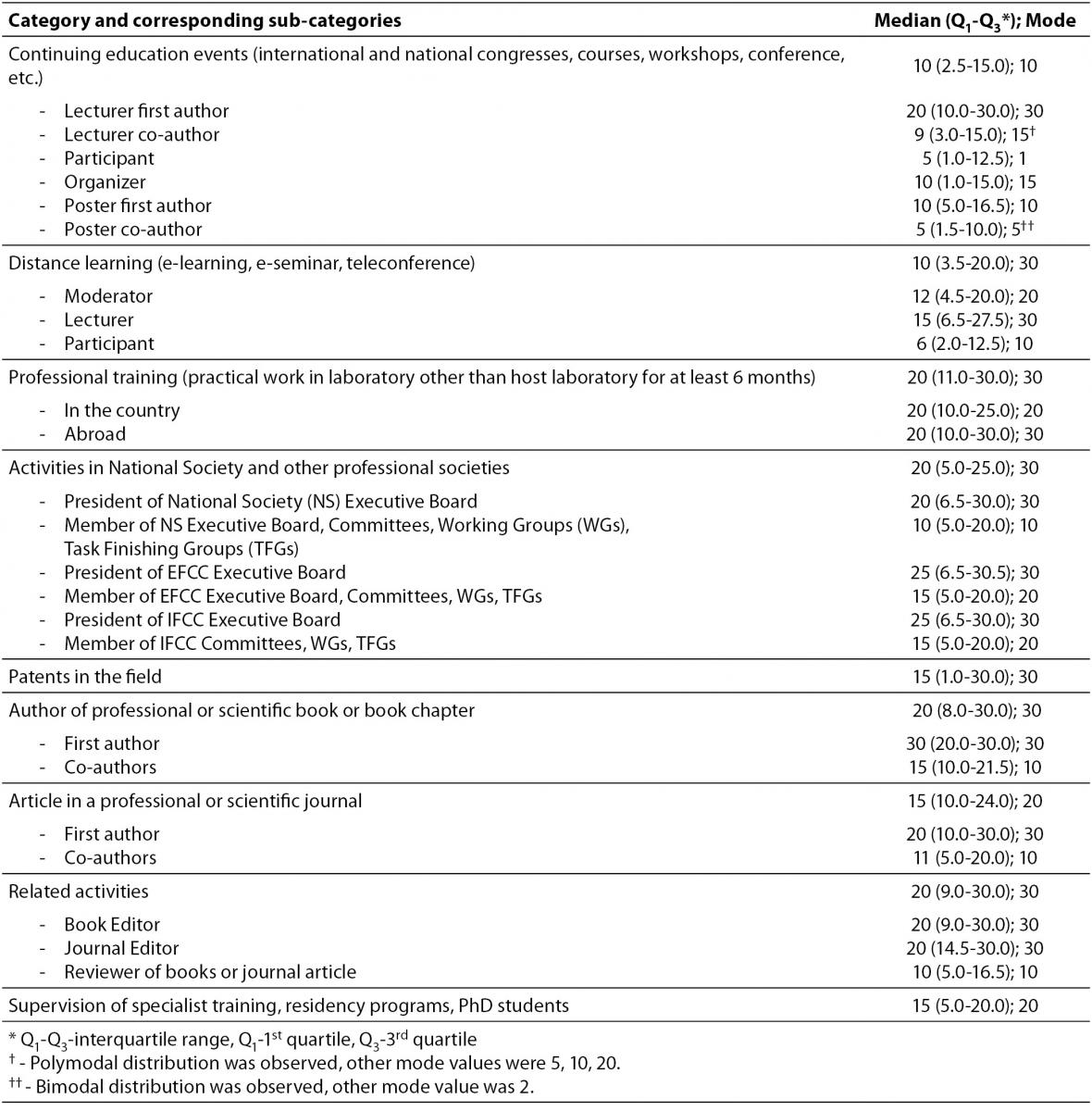
Table 6 contains scores allocated to 9 categories and 25 belonging sub-categories of CPD activities, according to appreciation of their importance. With the highest median score (20) were evaluated professional training, authorship of professional or scientific book or book chapter, activities in national society and other professional societies and engagement as book or journal editor. Among these categories professional training showed the least variation, presented as interquartile range among scores (IQR). Professional training was the only category showing no differences among corresponding sub-categories. Calculated as mode, the maximum of 30 points was the score with the highest frequency for all the categories except continuing education events, article in a professional or scientific journal and supervision of specialist training, residency programs and PhD students.
Table 6. Scores allocated to CPD categories and sub-categories, in accordance with appreciation of their importance.
Discussion
Up to our knowledge, the first integrative insight into CPD situation and corresponding crediting system for the profession specialist in laboratory medicine among European countries is provided by the presented results. It demonstrated that most of EFLM member societies have CPD programmes and corresponding crediting systems, indicating that European Specialists for Laboratory Medicine recognize CPD as one of the fundamental principles in their activities.
For EFLM member countries with no organized CPD system, current results might serve as a basis for further surveys attempting to evaluate and “bridge-over” educational gap. In addition, the survey results reinforce the fact that professionals with different basic academic background have the unequal availability for CPD. Consequently, the activities to ameliorate this “bias” should represent one of the key action points in further professional harmonization of laboratory medicine across Europe (7,8). The EC4 European Syllabus for Postgraduate Training in Clinical Chemistry and Laboratory Medicine: version, published in 2012, represents a valuable guideline for planning future activities to improve these inconsistencies. The syllabus is an attempt to upgrade knowledge harmonization of Specialist in Laboratory Medicine and to guide creation of national programmes (9).
All participating countries, regardless of CPD system establishment, reported on regular evaluation of CPD events, thus providing satisfactory confidence in quality of these activities. Nevertheless, some potential doubt is introduced with the finding that the evaluation authority is not uniformly defined. In the most of participating countries evaluation of CPD is conferred to a group of different institutions jointly termed as “another organisation”, including Social Security System, Cyprus Medical Association, Regional Accredited CME providers (including NS), Regional Organizations or Accredited CME providers, Slovak Accreditation Council for Continuing Education (SACCME), Non-profit Organization accredited by Government, Employer etc. On the other side, there are countries which rather systematically arranged this area, by appointing professional and governmental bodies, or national societies as responsible for this process.
Results of the conducted survey also reveal the differences in the process of registration and re-registration. Even in 22 countries, laboratory specialists should be registered to work in medical laboratories, although the validity of a registration cycle shows big differences. The re-registration conditions show even higher extent of variability, as illustrated by the finding that in even almost half of countries the re-registration is not required. The reason of such discrepancy might be that in some countries there is life-long registration validity, while on the other side the registration is still voluntarily in some countries. However, re-registration has been recognized as a prerequisite since the first attempts to establish rules for specialist in laboratory medicine, so that it is officially recommended to be repeated each 5 years (3,7). Although EC4 recognized CPD as prerequisite for re-registration/licensing, this survey revealed that this recommendation is implemented in less than a half of EFLM member countries. This finding suggests that for the unhampered harmonization activities might be necessary to include internationally recognized organisations accountable for continuing quality improvement and safeguarding of high standards in clinical laboratory practice (10).
The data from the survey confirmed that CPD credits can be collected through participation in various categories of CPD activities. Although the differences in representation of various categories are rather obvious, it should be emphasized that continuing education is recognized as essential CPD element in most EFLM member countries. Usually, it is accompanied by authorship, distance learning and activities in professional societies. It is sure that the number of CPD categories can be increased due to new educational tools as well as new achievements of specialist in laboratory medicine. Consequently, the CPD programme structure should always cover a wide range of activities intended to CPD, thus offering substantial benefits, like strengthening the position of the laboratory medicine at the clinical interface (11).
The survey provided an insight into judgment of importance that individual categories have for successful CPD. Although two types of statistical values were used for description, precise and subtle differences were identified by median and interquartile range. The equal highest score was allocated to professional training, activities in National Society and other professional societies, authorship of professional or scientific book or book chapter and book/journal editorship. However, professional training should be appreciated as the most important because it is associated with the least variation presented by IQR. Patents in the field, article authorship and mentoring share similar relevance. It was observed that CME and distance learning programmes, developed much later, have equal importance which is far lower than professional training relevance. This might indicate that EFLM member societies recognize and accept contemporary forms of education as more appropriate for comprehensive CPD programmes. Additional implication can be derived from significant variation among sub-categories belonging to continuing education events and distance learning. The lowest scores that subcategory “participation” earned infers this activity, being passive in its essence, is not regarded as a progress in CPD. In contrast, any activity associated with personal involvement (i.e. preparation of a poster or a lecture) is granted by significantly higher score. Differences among other sub-categories were expected.
The survey did not address the techniques of CPD evaluation. However, the most articles report about CPD among physicians, with the evaluation mostly based on hours. Very seldom, such data can be found for specialist in laboratory medicine, as it is the case in the UK where the Royal College of Pathology uses an approved CPD protocol (12). Accordingly, over 5 years participants should complete and record a minimum of 250 CPD hours, structured into three categories (clinical, academic & professional and a wide range of other activities applicable for CPD) (13). Croatian Chamber of Medical Biochemists evaluates 16 different categories. Members are required to annually record a minimum of 30 credits (approx. 50 hours) from different sources, in order to be eligible for re-registration, which is demanded every 6 years. The CPD structure has a significant extent of analogy with the UK model (14). Very similar protocols are applied in Serbia and Czech Republic. Bylaws issued by Serbian Health Council, the governmental body responsible for CPD of all health care providers, are used to evaluate CPD events, while the collected credits are annually reported to Serbian Chamber of Biochemists, the institution responsible for licensing. A minimum of 24 credits is required per year, while the validity of the registration cycle is 7 years (15,16). The credits for non-MD specialists are according the law No 96/2004 and announcement of Ministry of Health No 4/2010 in Czech Republic. The announcement is describing the different activities connecting to granting credits – lectures, courses, publications etc. The credits for MD specialist are granted according the professional regulation No 16 of Czech Medical Chamber (17,18).
Additional limitation of the survey is that some of rather important issues like institutions responsible for different issues in CPD programmes are not covered by the questionnaire (i.e. institutions responsible for specialists’ registration, prevention of participants’ misconduct, legal status of registrants etc). The assumption of personal bias in scoring CPD categories’ importance is also rather justifiable.
The obtained results suggest the next step for EFLM in this field, which is to create its own CPD programme based on recognition and evaluation of current National CPD programmes. Its goal should be dual: to stimulate learning and facilitate changes in laboratory practice behaviour. Considering time framework for CPD, its audit should be based on time-cycles of reasonable duration (3-5 years) during which professionals can earn credits from at least three different categories. In addition, EFLM should make agreements based on recognition of credit points with the National Societies and National Bodies. An example of such collaboration is agreement with UEMS (19-21).
It is recommended for the audit report of CPD events to include following items: name of the educational event provider, data about organization responsible for evaluation of the programme, names of educators, learning styles, assessment and appraisal of the events. The responsibilities of an individual laboratory professional should be to achieve agreed educational objectives, ensure required number of CPD hours and submit educational credits annually to the National Society which should perform annual appraisal and final audit to EFLM (22).
It is reasonable to expect that optimal usage of current EFLM CPD sources will significantly facilitate upcoming activities. Concerning corresponding terms of reference, the greatest efforts are issued by EFLM Education and Training Committee (C-ET), integrating two working groups (WG): WG on Congresses and Postgraduate Education and WG on Distant Education Programs and e-Learning. At the moment EFLM C-ET appointed 3 Educational Centres as organizers of educational and scientific events: Dubrovnik (Croatia) for EFLM Continuous Postgraduate Course in Clinical Chemistry and Laboratory Medicine and Belgrade (Serbia) for EFLM Symposium for Balkan Region, which are organized annually (23-25), together with Prague (Czech Republic) for EFLM Symposium on Education. First activities in the field of distance learning started in 2011 in form of e-seminars which served as a source of CPD on-line education to EFLM members. The WG for distance learning/e-learning recently published a position paper of the EFLM Committee on Education and Training and Working Group on Distance Education Programmes/ E-Learning: developing an e-learning platform for the education of stakeholders in laboratory medicine (26). In the near future additional EFLM educational conferences are expected to be established.
Conclusion
Presented results provide enough confidence to conclude that the majority of EFLM members have developed CPD programmes, regularly evaluated and accompanied by crediting systems. Programmes differ in accessibility for non-medical scientists and impact on relicensing eligibility. Continuing education, authorship and e-learning are mainly recognized as CPD activities, although the professional training is appreciated as the most important individual CPD category.
Acknowledgment
Authors thank to all National Societies which filled in the questionnaire and also the members of C-ET and WG C-ET for their support and constructive discussion. The authors thank to Silvia Cattaneo, the EFLM administrative secretary, for technical assistance in creating the survey.