Burden of disease
Neonatal sepsis (NS) is single most important cause of neonatal deaths in the community, accounting for over half of them (1,2). If diagnosed early and treated aggressively it is possible to save most cases of NS (3). NS is defined as a clinical syndrome characterized by signs and symptoms of infection with or without accompanying bacteremia in the first month of life. It encompasses various systemic infections of the newborn such as septicaemia, meningitis, pneumonia, arthritis, osteomyelitis etc., but it does not include superficial infections like thrush (4). Neonatal sepsis may be defined, both clinically and/or microbiologically, by positive blood and/or cerebrospinal fluid cultures (5,6). The incidence of culture-proven sepsis is approximately 2 per 1,000 live births. There is no universal agreement on definition of neonatal sepsis, according to various diagnostic criteria, incidence of neonatal sepsis in developed countries is 2.2-8.6 per 1,000 live births (7-9). Babies in the neonatal intensive care units (NICU) are at increased risk for acquiring nosocomial (hospital-acquired) infections. Many babies in the NICU are premature or have low birthweight which makes them more susceptable to infection and more likely to need invasive treatments and procedures. Nosocomial blood stream infections continue to be a cause of high mortality and morbidity in NICUs(10).
Early vs. late sepsis
Neonatal sepsis can be classified into two sub-types depending upon whether the onset of symptoms is before 72 hours of life (early onset) or later (late onset). Early-onset infections are caused by organisms prevalent in the maternal genital tract or in the delivery area. Risk factors for early-onset sepsis include prematurity, low birth weight, premature and prolonged rupture of membranes, maternal fever, uroinfection and chorioamnionitis.
Late-onset sepsis (LOS) is caused by the organisms thriving in the external environment of the home or the hospital. The infection is often transmitted through the hands of the care-providers. The onset of symptoms is usually delayed beyond 72 hours after birth and the presentation is that of septicemia, pneumonia or meningitis. LOS is a common complication of prolonged admission to the NICU following preterm birth (11). Risk factors for LOS are invasive procedures such as resuscitation in delivery room, intubation, mechanical ventilation, central venous catheters, surgical procedures and staying in NICUs for prolonged period of time. The use of broad spectrum antibiotics is risk factor for fungal NS.
Causative agents of sepsis
The microorganisms most commonly associated with early onset infection include group B Streptococcus (GBS), Escherichia coli, coagulase negative Staphylococci (CONS), Haemophilus influenzae and Lysteria monocytogenes (12).
Group B streptococcus arised as an important cause of neonatal and maternal pathogen in USA & Western Europe with reported mortality rate of 15-50% (13). In the USA 10-35% of pregnant women are asymptomatic carriers of GBS in genital and gastro intestinal tract at the time of delivery (14). Approximately 98% of colonized newborn remain healthy but 1-2% develop invasive GBS infection. The overall incidence of neonatal GBS infection was approximately 2/1,000 live births in the USA prior to introduction of intra-partum prophylaxis. Trends in the epidemiology of early onset sepsis show a decreasing incidence of GBS sepsis (15). After introduction of intrapartal prophylaxis against GBS infection there was a significant decline in early GBS NS in newborns less than 1500 g birthweight (16). GBS remains the most frequent significant pathogen in term infants and E. coli the most significant pathogen in preterm infants (17).
Late onset sepsis syndrome occurs at 7-90 days of life and is acquired from the caregiving environment. Organisms that are associated with late onset sepsis are Staphylococcus aureus, CONS, E. coli, Klebsiella, Pseudomonas, Enterobacter, Candida, GBS, Serratia, Acinobacterand anaerobes. Trends in late-onset sepsis show an increase in CONS sepsis (17). Late onset disease has a higher case fatality rate when gram negative bacteria are involved.
Susceptability for neonatal infection
The fetus has some preformed immunoglobulin present, primarily acquired through nonspecific placental transfer from the mother. Most of this transfer occurs in late gestation, such that lower levels are found with increasing prematurity. The neonate may receive immunoglobulin A (IgA) from breastfeeding but does not secrete IgA until 2-5 weeks after birth. Concentration of various components of the complement system widely varies among individual neonates. The terminal cytotoxic components of the complement cascade that leads to killing of organisms, especially gram-negative bacteria, are deficient. Natural killer (NK) cells are found in small numbers in the peripheral blood of neonates. These cells are also functionally immature in a way that they produce far lower levels of interferon-gamma upon primary stimulation than do adult NK cells. The physical and chemical barriers to infection in the human body are present in the newborn but are functionally deficient. Skin and mucus membranes are broken down easily in premature infants.
Clinical features
Signs of sepsis in neonates are often non specific (Table 1). It is important to recognise signs and symptoms of possible infection to make early diagnosis. A neonate with sepsis may present with signs and symptoms of hypothermia or fever, poor cry, poor feeding or refusal to suck, lethargy, hypotonia, respiratory distress, apnea, fast breathing, poor perfusion, hypoglycaemia and prolonged capillary refill time. Meningitis is often silent, the clinical picture is dominated by manifestations of associated septicemia.
Table 1. Clinical manifestations of neonatal sepsis.
The evaluation test for neonatal sepsis is important because infection indicates a very serious threat to babies. There is no single test that can be confidently used and applied by physicians/neonatologists to predict neonatal sepsis. Most of the diagnostic tests are not available in secondary and primary care level. So the diagnostic approach focuses on history and review of non specific signs and symptoms (18,19).
Diagnostic tools for neonatal sepsis
The isolation of microorganisms from blood, cerebrospinal fluid (CSF) or urine remains the gold standard for definitive diagnosis, however, confirmation or exclusion of positive cultures requires days and the sensitivity of the culture methods is frequently low because of the concomitant antibiotic therapy, small blood sample volume or low colony counts. In addition, clinical signs of sepsis in neonates are poor, late and non specific, particulary in preterm infants, in whom the onset of sepsis may be acute and clinical course can quickly deteriorate.
If possible, lumbar puncture should be done in all cases of late onset (> 72 hours) and symptomatic early onset sepsis because 10-15 percent of children may have associated meningitis. Detecting meningitis in septicemia is important because of the need for using antibiotics with a high CSF penetration and provision of antibiotic treatment for at least 3 weeks parenterally.
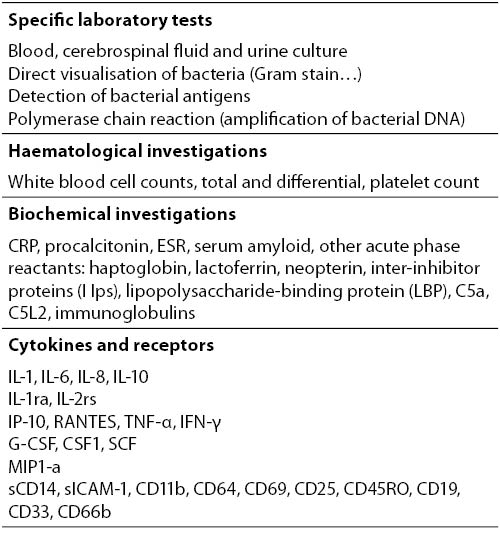
Nonspecific laboratory investigation for the diagnosis of invasive bacterial infections remain the most important diagnostic aid for the management of septic neonates (20) (Table 2).
Table 2. Laboratory investigations for diagnosis of neonatal sepsis.
The role of laboratory results and their notification of critical values can sometimes be crucial in the management of the patient. Therefore, all efforts need to be made in order to establish the appropriate link between laboratory and the clinic in terms of the timely notification of the critical values for a critically ill patient. The criteria for considering test results critical is controversal (21). There are a variety of tests which are helpful for screening of neonates with sepsis. The most useful and widely used is the white blood cell count and differential count. Neutropenia is more predictive of neonatal sepsis than neutrophilia, but it may be present in maternal hypertension, birth asphyxia and periventricular hemorrhage. Total leukocyte count (> 20 and < 5 x 109 / L), differential leukocyte count and morphology, total neutrophil count, total nonsegmented neutrophil count, neuotrophil ratios, platelet count are the indices most commonly used. These haematological counts and ratios showed a limited accuracy with wide range of sensitivity (17-90%) and specificity (31-100%), due to the relatively long period necessary to become positive and the significant influence of non-specific factors. However, the bands neutrophil: total neutrophil (IT) ratio of > 0.2 may reach a sensitivity of 90% and the negative predictive value of 98%. The IT ratio is less influenced by non infectious factors, as the method of delivery (22). Platelet count of less than 100 x 109 / L can be indirect evidence of infection.
C-Reactive Protein (CRP) is a globulin that forms a precipitate when combined with the C-polysaccharide of Streptococcus pneumoniae. It is the most extensively acute phase reactant studied so far. The sensitivity is low for early diagnosis of sepsis, due to the relatively long time, 6-8 hours following the stimulus, required for the synthesis; the peak is observed at 24 hours. The qualitative assay of CRP does not offer significant advantages on the leukocyte indexes. On the other hand, quantitative CRP values, particularly when repeated, are highly specific and have good sensitivity. In addition, serial measurements can be helpful in monitoring the response to treatment. In spite of the reduced early sensitivity, CRP still remains the preferred index in most neonatal ICUs.
Very high serum procalcitonin levels are present in neonates with proven or clinically diagnosed bacterial infection; early decrease of these concentrations reflects appropriate antibiotic therapy (23). Compared with CRP, procalcitonin has the advantage that increases more rapidly. However, in comparison to the CRP it’s usage has been limited because of significant rapid variations of basal levels after birth, and the need for several different cut-off values with changing neonatal age (24). Recent meta-analysis suggested that procalcitonin showed better accuracy than the CRP test for the diagnosis of late-onset sepsis (25). PCT concentration showed better discrimination than other inflammatory parameters for differentiating systemic from localised bacterial infection in critically ill patients (26). Procalcitonin should be used as an adjunct, rather than a replacement, to CRP to improve the diagnostic accuracy.
An important limit of haematological indices for early diagnosis of sepsis is the time required for the test to become positive. It takes several hours for leukocyte indices and acute-phase reactants to change significantly after the onset of reaction.
Several cytokines and receptors have been evaluated for the early diagnosis of infection in neonates (27-32). In most cases of neonatal sepsis, interleukin-6 (IL-6) increases rapidly, several hours before the increase in the concentration of CRP, and decreases within 24 hours to undetectable levels. IL-6 has good sensitivity and good specificity when used as a very early marker. A study showed that IL-6 and interleukin-1 receptor antagonist (IL-1ra) increased significantly two days before clinical diagnosis of sepsis (27). In contrast to CRP, IL-6 is very early marker but levels can become normal even if infection continues. When used in combination with CRP, the sensitivity is high for infected infants at any postnatal age.
Treatment
No investigation is required to start treatment in a clinically obvious case. Early treatment is crucial. Supportive care and antibiotics are two equally important components of the treatment. It should be realized that antibiotics take at least 12 to 24 hours to show any effect and it is the supportive care that makes the difference between life and death early in the hospital course. The purpose of supportive care is to normalize the temperature, stabilize the cardiopulmonary status, correct hypoglycemia and prevent bleeding tendency.
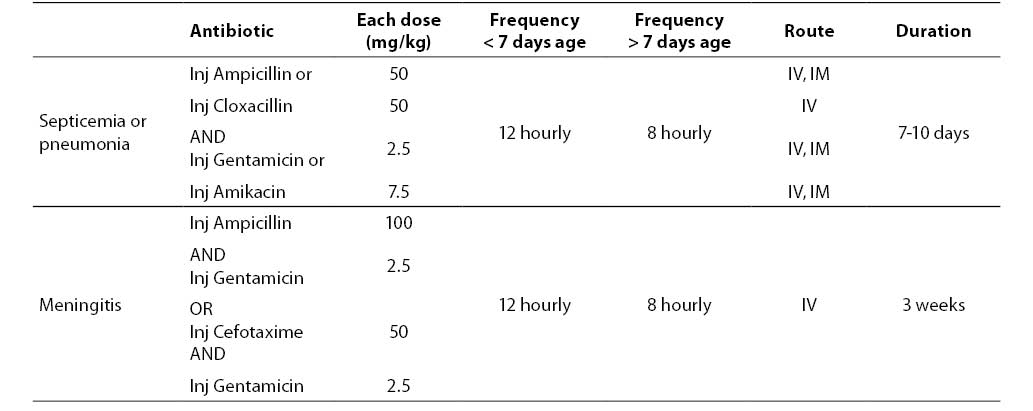
There is no single recommendation for antibiotic regimen for neonatal sepsis for all settings. Generally the choice of antibiotic depends on suspected flora in the given set up and their antimicrobial susceptibility. Antibiotic therapy is started when there is strong suspicion of sepsis. Choice of antibiotic also depends on source of infection. A combination of ampicillin or penicillin with gentamicin may be used as first line of therapy for community acquired infection where resistant strains are less likely. For hospital acquired infection where resistant strains are more likely a combination of ampicillin or cloxacillin and gentamicin or amikacin may be used (33).
Antibiotic therapy should cover the common causative bacteria - Escherichia coli, Staphylococcus aureus and Klebsiella pneumoniae. A combination of ampicillin and gentamicin is recommended for treatment of sepsis and pneumonia. In cases of suspected meningitis, cefotaxime should be used along with an aminoglycoside. Table 3 shows guidelines about antibiotic therapy. The duration of antibiotic therapy in sepsis depends upon the pathogen, site of infection and the clinical response of the baby. 7-10 days therapy is required for soft tissue infections or pneumonia. Deep-seated infections (osteomyelitis) and meningitis may require therapy for 3-6 weeks.
Table 3. Antibiotic therapy of neonatal sepsis.
Prevention of infections
A good antenatal care is important in decreasing the incidence, morbidity and mortality from neonatal sepsis. All types of infections should be diagnosed early and treated vigorously in pregnant mothers.
Conclusion
In conclusion, manifestations of neonatal sepsis are non-specific. A high index of suspicion with or without lab evidences of infection is the key for early diagnosis. Prompt institution of antibiotic therapy and supportive care will save most of the cases of neonatal sepsis.
Notes
Potential conflict of interest
None declared.
References
1. Bang At, Bang RA, Baitule SB, Reddy MH, Desmukh MD. Effect of home based neonatal care and management of sepsis on neonatal mortality: Field trial in rural India. Lancet 1999; 354:1955-61.
2. Stoll BJ. The global impact of neonatal infection. Clinical Perinatol 1997;24:1-21.
3. Management of neonatal sepsis, IAP-NNF (Indian Academy of Pediatrics – National Neonatology Forum) Guidelines 2006 on level II neonatal care: 159-86.
4. Shankar MJ, Aggarwal R, Deorari AK, Paul VK. Symposium on AIIMS protocol in Neonatology- III. Indian J Paediatr 2008;75:261-6.
5. WHO. Handbook IMCI Integrated Management of Childhood Ilnesses. WHO/FCH/CAH/00. 12 Geneva: WHO 2000.
6. Weber MW, Carlin JB, Gatchalian S, Lehmann D, Muhe L, Mulholland EK, et al. Predictors of neonatal sepsis in developing countries. Pediatr Infect Dis J 2003;22:711-6.
7. Tessin I, Trollfors B, Thiringer K. Incidence and etiology of neonatal septicaemia and meningitis in western Sweden 1975-1986. Acta Paediatr Scan 1990;79:1023-30.
8. Hervás JA, Alomar A, Salvá F, Reina J, Benedi VJ. Neonatal sepsis and meningitis in Mallorca, Spain, 1977-1991. Clin Infect Dis 1993;16:719-24.
9. Persson E, Trollfors B, Brandberg LL, Tessin I. Septicaemia and meningitis in neonates and during infancy in the Goteborg area of Sweden. Acta Paediatr 2002;91:1087-92.
10. Haque KN, Khan A, Kerry S, Stephenson J, Woods J. Pattern of culture proven neonatal sepsis in a district general hospital in the UK. Inf Control Hosp Epidemiol 2004;25:759-64.
11. Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late- onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 2002;110:285–91.
12. Klinger G, Levy I, Sirota L, Boyko V, Reichman B, Lerner-Geva L. Epidemiology and risk factors for early onset sepsis among very-low-birthweight infants. Am J Obstet Gynecol 2009;201:38.e1-6.
13. Baker CJ, Edwards MS. Group streptococcal infections. In Remmington JS, Klein JO, eds. Infectious diseases of the fetus and newborn infant. Philadelphia: WB Saunders; 2001.p.1051-6.
14. Regan JA, Klebanolf MA, Mugent RP. The epidemiology of Group B streptococcal colonization in pregnancy. Vaginal infections and prematurity study group. Obst Gynaecol 1991;77:604-10.
15. Van den Hoogen A, Gerards LJ, Verboon-Maciolek MA, Fleer A, Krediet TG. Long-Term Trends in the Epidemiology of Neonatal Sepsis and Antibiotic Susceptibility of Causative Agents. Neonatology 2009;97:22-8.
16. Schrag SJ, Zywicki S, Farley MM, Reingold AL, Harrison LH, Lefkowitz LB, et al. Group B streptococcal disease in the era of intrapartum antibiotic prophylaxis. N Engl J Med 2000;342:15-20.
17. Stroll BJ, Hansen NI, Sanchez PJ, Faix RG, Poindexter BB, Van Meurs KP, et al. Early onset neonatal sepsis: the burden of group B Streptococcal and E.coli diseasecontinues. Pediatics 2011;127:817-26.
18. Nyhan Wl, Fousek MD. Septicaemia of the newborn. Paediatrics 1958;22:268-78.
19. Philip AGS, Hewiot JR. Early diagnosis of neonatal sepsis. Paediatrics 1980;65:1036-41.
20. Benitz WE. Adjunct laboratory tests in the diagnosis of early-onset neonatal sepsis. Clin Perinatol 2010;37:421-38.
21. Plebani M, Piva E. Quality in laboratory diagnostics: from theory to practice. Biochem Med 2010;20:173-8.
22. Chirico G, Gasparoni A, Ciardelli A, Martinotti L, Rondini G. Leukocyte counts in relation to the method of delivery during the first five days of life. Biol Neonate 1999;75:294-9.
23. Mussap M, Degrandi R, Cataldi L, Fanos V, Plebani M. Biochemical markers for the early assessment of neonatal sepsis: the role of procalcitonin. J Chemother 2007;19:35-8.
24. Lapillonne A, Basson E, Monneret G, Bienvenu J, Salle BL. Lack of specificity of procalcitonin for sepsis diagnosis in premature infants. Lancet 1998;351:1211-2.
25. Yu Z, Liu J, Sun Q, Qiu J, Han S, Guo X. The accuracy of the procalcitonin test for the diagnosis of neonatal sepsis: a meta-analysis. Scand J Infect Dis 2010;42:723-33.
26. Pavic M, Bronic A, Milevoj Koprcinovic L. Procalcitonin in the systemic and localized bacterial infection. Biochem Med 2010;20:236-41.
27. Berner R, Niermeyer CM, Leititis JU, Funke A, Schwab C, Rau U, et al. Plasma levels and gene expression of granulocyte colony-stimulating factor, tumor necrosis factor- alpha, interleukin (IL)-1beta, IL-6, IL- 8, and soluble intercellular adhesion molecule-1 in neonatal early onset sepsis. Pediatr Res 1998;44:469-77.
28. Buscher U, Chen FC, Pitzen A, Menon R, Vogel M, Obladen M, et al. IL-1 beta IL-6, IL-8 and G-CSF in the diagnosis of early- onset neonatal infections. J Perinat Med 2000;28:383-8.
29. Ng PC, Lam HS. Biomarkers for late-onset neonatal sepsis: cytokines and beyond. Clin Perinatol 2010;37:599-610.
30. Kantar M, Kultursay N, Kutukculer N, Akisu M, Cetingul N, Caglayan S. Plasma concentrations of granulocyte-macrophage colony-stimulating factor and interleukin-6 in septic and healthy preterms. Eur J Pediatr 2000;159:156-7.
31. Krueger M, Nauck MS, Sang S, Hentschel R, Wieland H, Berner R. Cord blood levels of interleukin-6 and interleukin-8 for the immediate diagnosis of early- onset infection in premature infants. Biol Neonate 2001;80:118-23.
32. Bhandari V, Wang C, Rinder C, Rinder H. Hematologic profile of sepsis in neonates: neutrophil CD64 as a diagnostic marker. Pediatrics 2008;121:129-34.
33. Shankar MJ, Aggarwal R, Deorari AK, Paul VK. Symposium on AIIMS protocol in Neonatology- III. Indian J Paediatr 2008;75:261-6.