Introduction
Winter swimming (WS), is a cold-based activity practised during wintertime in frozen rivers, lakes or sea and, it is mainly practised in Northern countries that are characterized by long winters and low temperature averages (1). WS is rarely performed in Mediterranean countries, although in some regions a continental climate is dominant, including very low winter temperatures and icing of lakes and rivers. In these countries WS is considered an “extreme” sport, since the high level of psychophysical stress due to the contact and physical activity in cold water universally realised by general population, but also by athletes.
Other than the recreational aspect of this activity, WS and the whole-body cryotherapy treatment are peculiar cold-based procedures for fitness able to improve general well-being, but they are also considered to be effective in reinforcing against respiratory tract infections and relieving from musculoskeletal pains (2). Indeed cold-based procedures have long been used to relieve pain and inflammatory symptoms, to reduce muscular discomfort and to improve recovery following muscle traumas accounting for the intrinsic analgesic effects (3,4), although an universal acceptance as a real therapy is not still achieved.
Between the natural factors, water is the most effective thermal exchanger because of the relatively high coefficient of thermal conductivity. However, the conditioning efficiency depends on physical characteristics, intensity and duration of the exposure (5).
We have previously demonstrated that whole-body cryotherapy did not enhance haematological values, as judged from haemoglobin concentrations and the number of erythrocytes, reticulocytes, leukocytes, and platelets (6); the treatment was beneficial for muscle recovery following regular training and, at the same time, it induced a decrease in pro-inflammatory cytokines/chemokines and an increase in anti-inflammatory cytokines (7). Moreover, cryotherapy was reported not to have adverse effect on lung function (8), and did not decrease the antioxidant capacity (1). These results clearly showed the usefulness of the cold-based treatment in improving the sense of general well-being without any discomfort or disturbance.
As regard the WS, an increase of resting concentrations of leukocytes, monocytes and plasma IL-6 in regular winter swimmers, compared with inexperienced, subjects has been described, following the application of consecutive thermal stresses (sauna and WS) (9). Moreover, after exposure to the consecutive thermal stresses, the capacity of isolated blood mononuclear cells to produce IL-1beta and IL-6 was significantly suppressed in inexperienced subjects, but tended to increase in regular winter swimmers indicating that habitual WS slightly stimulates the immune system, leading to speculation that they are more prepared to react to an infection (9).
Long-term WS does not stimulate the pituitary-adrenal axis, although an habituation to the stressful episodes induces a decrease of ACTH (10). The pain alleviation, which is a common effect of cold exposure, could be linked to the increase of norepinephrine, which is not accompanied by an increase of epinephrine (10).
To our knowledge only few works have investigated the effects of cold-based activities/treatments on haematological parameters (5,6,11-14) and among them none have investigated the acute effects of a single session of WS, in non-habitual winter swimmers, on the haematological asset.
In this study we have evaluated haematological effects of a single session of WS performed by volunteer swimmers into a channel at 6 °C in January, in Milan, Italy, in order to verify the possible dangerous and beneficial modifications induced by body adaptation to physical exercise in a cold environment in non professional athletes.
Since the WS is becoming a popular recreational activity in temperate countries, other than in northern countries, with this study we aimed to define the physiological responses, and thus eventual detrimental responses, of the “blood system” to this strong cold stress, in people approaching WS for the first time.
Materials and methods
Subjects
Fifteen subjects were voluntarily recruited among the participants to “Cimento 2009”, a traditional non-competitive 150 meters long swimming performance, taking place from 1916, into the Naviglio channel in Milan. On January the 27th, at 11:00 a.m., during the performance, the water temperature was 6 °C, while the air temperature was 5°C.
An informed consent was obtained from each swimmer according to international standards and following the ethical standards reported in the paper of Harriss and Atkinson (15).
The recruited cohort was composed of 13 males and 2 females (age range: 21–58, median age 41 years). The health status of the participants was checked by sport physicians the day before the performance. All the participants were asked to submit to a medical interview to exclude the chronic assumption of prescribed medications and the presence of past or present relevant cardiovascular, infectious and respiratory diseases, and thus to asses the overall healthy status. All of them were non-professional rowers, performing no more than 2 hours of physical training, three times per week, and none of them have never been exposed to a such cold stress.
All the participants wore identical a bathing suit and a bathing cap and the exposure lasted 10 minutes. The intensity level of physical activity was submaximal.
Methods
The blood drawings were performed the day before and immediately after the performance (within 10 minutes), at the same time (11:00 a.m.), during both sessions of collection. Six mL of blood, per time, were drawn in two K2EDTA BD Vacutainers®, by antecubital venipuncture, from subjects under resting conditions and in a seated position. Blood collections were performed strictly following the preanalytical warnings (16): briefly, following the collection, samples were stored in a 4 °C cooled box, for transport, and haematological analysis were performed within an hour, on Sysmex XT 2100 (Sysmex, Tokyo, Japan). A single measurement was performed for each parameter at each timepoint. The haematological parameters measured were: haemoglobin (Hb; g/L), haematocrit (Ht; %), red blood cells count (RBC; 1012/L), platelet count (PLT; 109/L), white blood cells count (WBC; 109/L), automated differential blood count including relative (%) and absolute number (109/L) of leukocyte subpopulations, mean corpuscular haemoglobin (MCH, pg), mean corpuscular volume (MCV, fL) and mean corpuscular haemoglobin concentration (MCHC, g/L).
Statistical analysis
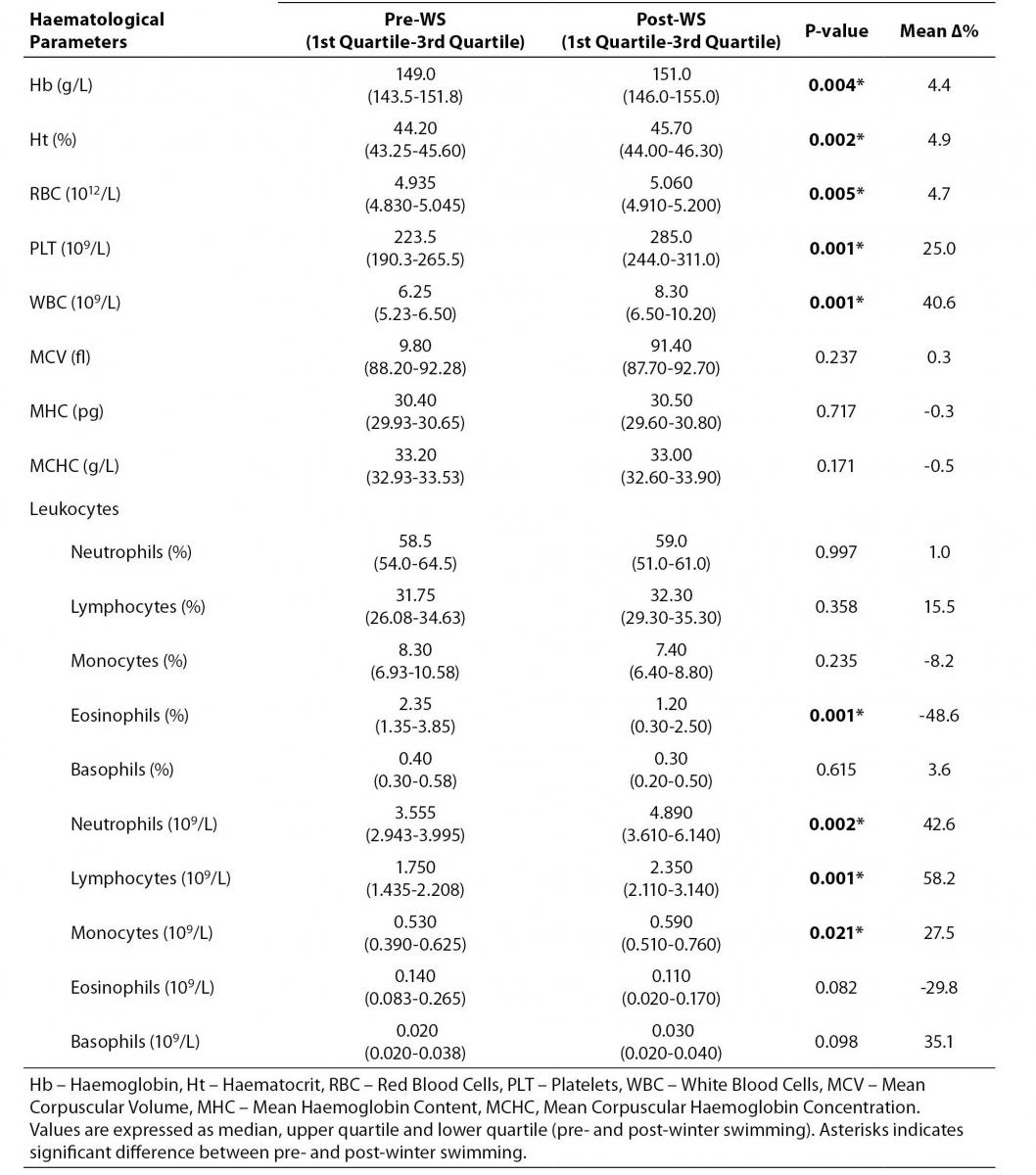
All values in the descriptive analysis are expressed as the median and interquartile range and reported in table 1.
Table 1. Winter swimming effects on haematological parameters.
The variations between the pre-WS and post-WS measurements were calculated, for each parameter and for each subject, as follows:
Δ%= 100 x (X2-X1)/X1
where:
- X2: value after the intervention
- X1: value before the intervention.
The mean Δ% was calculated as the mean between the subjects for each parameter.
The percentage of plasma volume change (ΔPV%), eventually due to the intervention, was calculated from the changes in values of haematocrit (Ht) and haemoglobin (Hb) according to the Dill and Costill’s formula (17) in Harrison modification (18):
ΔPV%= 100 x (Hb1 / Hb2) x {(100 - (Ht2 x 0.874)) / (100 - (Ht1 x 0.874))} - 1
where:
- Hb1: haemoglobin concentration before the intervention
- Hb2: haemoglobin concentration after the intervention
- Ht1: haematocrit before the intervention
- Ht2: haematocrit after the intervention.
All of the parameters were described by location (mean) and dispersion (standard deviation-interquartile range of the variation) indexes reported on Table 1. The modification attributable to the cold water exposure was evaluated by a Wilcoxon signed rank test (19) applied to the parameter difference, since the not normally distributed values within the population. A P-value lower than 0.05 was considered significant. Statistical analysis was performed using SAS software package vers. 9.1.3.
Results
As evident from the presented data, the cold-stress combined with the swimming activity induced a clear physiological response on haemopoiesis.
First of all we found a significant increase in both means Hb and Ht, 4.4% and 4.9% respectively. The total number of WBC significantly and strongly increased from (6.25 (5.23-6.50)) x 109/L to (8.30 (6.50-10.20)) x 109/L (Δ% = 40.6%), P = 0.001, along with a slight but significant increase of the RBC count: (4.935 (4.830-5.045)) x 1012/L; vs. (5.060 (4.910-5.200)) x 109/L; Δ% = 4.7%, P = 0.005. Remarkably, we have found a strong and, somewhat unexpected, increase of the PLT count from (223.5 (190.3-265.5)) x 109/L, at rest, to (285.0 (244.0-311.0)) x 109/L, immediately following the competition, with a mean percentage variation of 25.0% (P = 0.001) (Table 1).
While the relative number of leukocytes was not substantially affected by the WS activity, except for a steep decrease in the percentage of eosinophils (Δ% = -48.6%), P = 0.001, when considering the absolute number of leukocyte subpopulations, we found strong increases in the absolute number of neutrophils granulocytes (Δ% = 42.6%, P = 0.002), lymphocytes (Δ% = 58.2%, P = 0.001) and monocytes (Δ% = 27.5%, P = 0.021) (Table 1).
The swimming activity in cold water induced a decrease in plasma volume of 2.54%, calculated on the basis of Hb and Ht variations (17,18). Following normalization of all the parameters, on plasma volume change, the results were unchanged, demonstrating that the recorded variations were not due to a mere haemoconcentration.
No variations were found in the haematological indexes MCH, MCV and MCHC.
Table 1 illustrates median pre and post WS values and interquartile range, with the associated probability levels (P-values) and their mean percentage variations (Δ%).
Discussion
The question we aimed to answer was whether short-term submaximal physical exercise in cold water represents a stress factor affecting the haematological parameters in healthy non-habitual winter swimmers. Indeed, while a number of studies evaluated the effects of thermal stress (whole-body cryotherapy and WS) on sympathetic activity and inflammatory status (6,7,9,10,20-24), to our knowledge this is one of the few works on WS focusing on the effects of a short-term physical exercise in cold water on the blood cell composition.
In this work we found that mild intensity swimming in cold water (6 °C) induced significant variation in the blood cell fraction composition in respect to the rest condition, as measured the day before the competition. RBC, WBC and PLT all increased significantly (4.7%, 40.6% and 25.0% respectively). The consistent increase in the total number of WBC indicate a cold stress-induced generalized reactive leukocytosis since the relative number of leukocytes did not change significantly, apart from a strong decrease in the eosinophil population (-48.6%). Indeed we found a strong increase in the number of neutrophil granulocytes, lymphocytes and monocytes (42.6%, 58.2% and 27.5% respectively), while the variation in the number of cells within both the eosinophil (decrease) and the basophil (increase) population did not reach the significance.
Following the cold-bath we found increased the Hb concentration and Ht parallely (4.4% and 4.9% respectively). Although this WS-induced haemoconcentration, the variation of the haematological parameters here considered remained significant in respect to the rest condition. The plasma volume changes could be imputed to the shift of plasma water from the intra- to the extravascular compartments, due to the sympathetic system activation and the consequent reactive vasoconstriction (25). Indeed, cold stress is reported to increase the sympathetic activity (24): plasma levels of ACTH, b-endorphine and cortisol following single or multiple sessions of cryotherapy, were not changed, as well as plasma epinephrine, thus demonstrating that the pituitary-adrenal axis was not activated (10,26). Blood levels of norepinephrine, instead, have been shown to increase (5,20-24), explaining the superficial vasoconstriction and partially explaining the reported pain alleviating effects, at the spinal cord level (27).
Our data are in concordance with that obtained by Vogelaere et al. (14), in the only comparable work in literature, reporting that cold exposure (0 °C), at rest or during maximal and submaximal physical activity, increased the number of RBC, WBC and PLT, the Hb concentration and the Ht along with a significant haemoconcentration without affecting MCV, MHC and MCHC.
Animal studies demonstrated that cold exposure induces changes in both cellular and humoral aspects of immune function, including a reduction in natural killer (NK) cell count and cytolytic activity, a decrease in lymphocyte proliferation and, after several days, an enhanced production of proinflammatory cytokines (12). These data appear to be not comparable to the present results and that reported in the work of Vogelaere et al. (14); this discrepancy could be imputed to the prolonged period of cold exposure (2 hours-long exposure in (12) vs. 10 minutes in the present study) and the strong psychological stress due to the procedure.
The combined cold water (18 °C) and cold air (5 °C) exposures, following or not a warm air treatment, in the experimental work carried out by Brenner et al, demonstrated a reactive leukocytosis, mainly due to increase of lymphocyte, granulocyte and monocyte counts (12). The overall data, thus clearly indicated that strong cold stress stimulates the haemopoiesis within the bone marrow.
It is reported that such changes in blood cell fraction could be in part due to the reactive spleen contraction, a phenomenon which characterizes physical exercise especially when performed in stressful environmental conditions (i.e. cold water). The spleen contains 200 mL of densely packed blood cells and up to 50% of these are transferred to circulation during maximal exercise or apnoeic diving (28,29). Spleen volume changes after an increase in sympathetic activity are probably due to a passive collapse rather an active contraction. RBC are released from spleen within one minute after exercise, whereas platelets and granulocytes are exited after ten minutes (29). The effect of spleen contraction and, consequently, that of the transfer of cells and particles to circulation appear to be not different between trained and untrained individuals (29).
The main finding of our work is represented by the strong increase in platelet count in these inexperienced winter swimmers. Although there is a clear concordance in the RBC and WBC counts between our study and the work of Baković and coworkers, we also found a significant increase in PLT count, when no difference was described in subjects who experienced apnoea in cold (12 °C) water (28). Thus, spleen contraction is not the source of PLT increase in our non habitual winter swimmers. Moreover, it is reported that the PLT expulsion from the spleen should be linked to the exercise intensity (28): in our study, the exercise was stressful (since the environmental conditions), but not intense (submaximal).
Recent evidence emphasises the pivotal role of abnormal platelet homoeostasis in acute coronary artery diseases, myocardial infarction, unstable angina and stroke (30). Therefore the cold-induced PLT count increase, along with the cold-induced leukocytosis that is a well-known risk factor for ischemic vascular disease morbidity and mortality (31), could be potentially dangerous, increasing the risk of thrombosis especially in non-habituated people already at risk for cardiovascular events, who try the WS as recreational activity. It must be noted that in healthy cohort of subject of this study, however, the haematological values reached following the intervention remain always in the normal range.
In any case, functional studies on adhesion and aggregation of platelets should be performed for evaluating this hypothesis.
In scuba and apnea divers the immersion in cold water induced a platelet activation evaluated by surface expression of activation-dependent glycoproteins CD62p, CD63, and CD42a (11). However, the number of PLT was not modified. By contrast, in decompression sickness, when the PLT activity is enhanced, the count appeared decreased (13). The exercise effects on platelet aggregation and function in healthy individuals have been extensively examined (reviewed in 30), the evidence reported has been conflicting; the exact effects of exercise training on platelet activation and function is not clear.
The increased number of the whole blood cells set seems to be due to a direct induction of the haemopoiesis at the bone marrow level under the pull of the sympathetic activity, through the activation of a series of hormonal mediators, regulating the energy metabolism and the cold adaptation, and among them, leptin (31).
Conclusions
Winter swimming, when represented by brief exposure to cold water, induces strong non-pathological modifications of haematological parameters, with a strong increase of RBC, WBC and PLT counts, other than a significant haemoconcentration. The main implications of these results reside in two different ambits. Since the presented theoretically potential mild additive effect on pre-existing cardiovascular risk factors might be considered in occasional winter swimmers approaching this activity with recreational or therapeutic aims. On the other hand, in a wider view, the possible variations in the haematological parameters following cold water-based treatments, aimed to a quicker functional recovery in professional athletes, during the competitive season, might need more consideration within the antidoping evaluation.
Acknowledgments
The authors would like to thanks the “Club Canottieri Olona” for the availability and the logistic support.
Notes
Potential conflict of interest
None declared.
References
1. Dugué B, Smolander J, Westerlund T, Oksa J, Nieminen R, Moilanen E, Mikkelson M. Acute and long-term effects of winter swimming and whole-body cryotherapy on plasma antioxidative capacity in healthy women. Scand J Clin Lab Invest 2005;65:395-402.
2. Kauppinen K, Urponen H. The winter swimmer’s self-portrait. Icewater immersion as a form of self care. Arct Med Res 1988;47:4-12.
3. Bugaj R. The cooling, analgesic, and rewarming effects of ice massage on localized skin. Phys Ther 1975;55:11-9.
4. Harris E, McCroskery P. The influence of temperature and fibril stability on degradation of cartilage by rheumatoid synovial collagenases. N Eng J Med 1974;290:1-6.
5. Kalenova LF, Fisher TA, Suhovey JG, Basedin IM. Effects of short-term hypothermal and contrast exposure on immunophysiological parameters of laboratory animals. Bull Exp Biol Med 2009;147:617-20.
6. Banfi G, Krajewska M, Melegati G, Patacchini M. Effects of the whole body cryotherapy on haematological values in athletes. Brit J Sports Med 2008;42:558.
7.Banfi G, Melegati G, Barassi A, Dogliotti G, Melzi d’Eril G, Dugué B, Corsi MM. Effects of whole-body cryotherapy on serum mediators of inflammation and serum muscle enzymes in athletes. J Thermal Biol 2009;34:55-9.
8. Smolander J, Westerlund T, Uusitalo A, Duguè B, Oksa J, Mikkelsson M. Lung function after acute and repeated exposures to extremely cold air (-110 degrees C) during whole-body cryotherapy. Clin Physiol Funct Imaging 2006;26:232-4.
9. Dugué B, Leppänen E. Adaptation related to cytokines in man: effects of regular swimming in ice-cold water. Clin Physiol 2000;20:114-21.
10. Leppäluoto J, Westerlund T, Huttunen P, Oksa J, Smolander J, Dugué B, Mikkelson M. Effects of long-term whole-body cold exposures on plasma concentrations of ACTH, beta-endorphin, cortisol, catecholamines and cytokines in healthy females. Scand J Clin Lab Invest 2008;68:145-53.
11. Bosco G, Yang ZJ, Savini F, Nubile G, Data PG, Wang JP, Camporesi EM. Environmental stress on diving-induced platlet activation. Undersea Hyperbaric Med 2001;28:207-11.
12. Brenner IKM, Castellani JW, Gabaree C, Young AJ, Zamecnik J, Shephard RJ, Shek PN. Immune changes in humans during cold exposure: effects of prior heating and exercise. J App Physiol 1999;87:699-710.
13. Pontier JM, Blatteau JE, Vallée N. Blood platelet count and severity of decompression sickness in rats after a provocative dive. Aviat Space Environ Med 2008;79:761-4.
14. Vogelaere P, Brasseur M, Quirion A, Leclercq R, Laurencelle L, Bekaert S. Hematological variations at rest and during maximal and submaximal exercise in a cold (0°C) environment. Int J Biometeorol. 1990;34:1-14.
15. Harriss DJ, Atkinson G. Ethical standards in sport and exercise science research. Int J Sports Med 2009;30:701–2.
16. Banfi G, Dolci A. Preanalytical phase of sport biochemistry and haematology. J Sports Med Phys Fitness 2003;43:223-31.
17. Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J App Physiol 1974;37:247-8.
18. Harrison MH. Effects of thermal stress and exercise on blood volume in humans. Phys Rev 1985;65:149-209.
19. Wilcoxon F. Individual comparisons by ranking methods. Biometrics 1945;1:80-3.
20. Huttunen P, Lando NG, Meshtsheryakov VA, Lyutov VA. Effects of long-distance swimming in cold water on temperature, blood pressure and stress hormones in winter swimmers. J Therm Biol 2000;25:171-4.
21. Huttunen P, Rintamäki H, Hirvinen J. Effect of regular winter swimming on the activity of the sympathoadrenal system before and after a single cold water immersion. Int J Circumpolar Health 2001;60:400-6.
22. Janský L, Šrámek P, Šavlíková J, Ulincý B, Janáková H, Horký K. Change in sympathetic activity, cardiovascular functions and plasma hormone concentrations due to cold water immersion in men. Eur J App Physiol Occup Physiol 1996;74:148-52.
23. Leppäluoto J, Pääkkönen T, Korhonen I, Hassi J. Pituitary and autonomic responses to cold exposure in man. Acta Physiol Scand 2005;184:255-64.
24. Šrámek P, Šimečková M, Janský L, Šavlíková J, Vybíral S. Human physiological responses to immersion into water of different temperatures. Eur J Appl Physiol 2000;81:436-42.
25. Pendergast DR, Lundgren CEG. The underwater environment: cardiopulmonary, thermal, and energetic demands. J Appl Physiol 2009;106:276-83.
26. Smolander J, Leppäluoto J, Westerlun T, Oksa J, Duguè B, Mikkelsson M, Ruokonen A. Effects of repeated whole body cold exposure on serum concentrations of growth hormone, thyrotropin, prolactin and thyroid hormones in healthy women. Cryobiology 2009;58:275-8.
27. Gresele P, Bounameaux H, Arnout J, Perez-Requejo JL, Deckmyn H, Vermylen J. Thromboxane A2 and prostacyclin do not modulate the systemic hemodynamic response to cold in humans. J Lab Clin Med 1985;106:534-41.
28. Baković D, Eterović D, Saratljia-Novaković Z, Palda I, Valic Z, Bilopavlović N, Dujić Z. Effect of human splenic contraction on variation in circulating blood cell counts. Clin Exp Pharmacol Physiol 2005;32:944-51.
29. Baković D, Valic Z, Eterović D, Vuković I, Obad A, Marinović-Terzić I, Dujić Z. Spleen volume and blood flow response to repeated breath-hold apneas. J Appl Physiol 2003;95:1460-66.
30. El Sayed MS, Ali N, El Sayed Ali Z. Aggregation and activation of blood platelets in exercise and training. Sports Med 2005;35:11-22.
31. Coller BS. Leukocytosis and ischemic vascular disease morbidity and mortality: is it time to intervene? Arterioscler Thromb Vasc Biol. 2005;25:658-70.
32. Rayner DV, Trayhurn P. Regulation of leptin production: sympathetic nervous system interactions. J Mol Med 2001;79:8-20.