Introduction
There is a great heterogeneity between European countries in definition of the profession of clinical chemistry and laboratory medicine and the academic background of specialists in this discipline (1,2). Laboratory professionals across Europe may have their basic education in medicine, pharmacy or science. A postgraduate training in the European countries lasts from four to seven years, whereas the total number of years of education (academic education and post-graduate training) ranges from nine to thirteen. However, despite such heterogeneity, the profession is practiced at fully comparable level in most countries in Europe.
European Communities Confederation of Clinical Chemistry (EC4) has for many years been very actively devoted to improving the level of the clinical chemistry profession in European Union (3,4). Thanks to this very active group, there has been quite a lot of activity towards the harmonization and common regulation of the undergraduate and postgraduate education as well as the vocational training programs in clinical chemistry across EU countries (5-7). However, a little is known on the situation in some other non-EU countries.
The knowledge on the situation in individual countries gives insight into the level of education and training of professionals as well as about the level at which the profession is practiced. This information becomes especially interesting when specialists in clinical chemistry and laboratory medicine want to move from one country to another. While medical professionals enjoy automatic recognition in majority of the European countries, situation is rather different for other laboratory professionals (8).
The purpose of this paper is to review the current status of the standards of education and training of laboratory professionals as well as laboratory quality regulations and accreditation standards in Croatia.
Croatia is a relatively small non-EU country in southeast Europe with 4.6 million inhabitants covering an area similar to Slovakia. Medical biochemistry and Medical biochemist are terms commonly used in Croatia, equivalent to Clinical chemistry and Clinical chemist, respectively, in most European countries. Medical biochemistry is almost exclusively practiced by medical biochemists. There is only a small number of biotechnologists specialized in medical biochemistry and only few medical doctors (MD). There are 190 laboratories in Croatia, out of which there are 102 in a primary health care section, 60 are hospital laboratories and 28 are privately owned laboratories. Furthermore, there are 536 registered medical biochemists, out of which 51 hold a PhD degree, 8 of them being Assistant professors and 8 Professors. Currently, 175 (33%) of the registered professionals are specialists in medical biochemistry.
Medical biochemistry in Croatia comprises clinical biochemistry, hematology and coagulation, immunology, toxicology and therapeutic drug monitoring and endocrinology. Blood-banking, microbiology and cytogenetics are out of the scope of the clinical chemistry in our country.
Profession of medical biochemist – explicitly defined by the law
Medical biochemistry as a profession is an integral part of the health care system. As in most of the European countries (1), practicing the medical biochemistry in Croatia is also regulated through The Health Care Law, The Law of the Medical Biochemistry Profession and The Law of the State and Private Health Insurance. Furthermore, there are some other regulatory documents issued by the Croatian Chamber of Medical Biochemists, which will be mentioned later in this text.
The competent authority for all health employees in our country is the Ministry of Health. As of The Health Care Law, there are four essential university level personnel in health care: Master of Medical Biochemistry, Master of Pharmacy, Medical Doctor and Dentistry Doctor.
The Law of the Medical Biochemistry Profession defines the profession and its scope, competencies and responsibilities. It furthermore explicitly defines responsibility and scope of activities for the Croatian Chamber of Medical Biochemists. According to that law, only medical biochemists can be employed in the medical biochemistry laboratory. Licensed medical biochemists may work in the medical laboratory, whereas laboratories are headed by specialists. This is very much consistent with the situation in Europe, where laboratories are also mostly headed by specialists in clinical chemistry and laboratory medicine (1). To be head of the clinical laboratory in university hospitals in Croatia, one needs to have a minimum of PhD degree and a specialization in medical biochemistry.
Specialists in medical biochemistry are actively involved as consultants to the clinicians offering them guidance and advice in selecting the right test for the right patient. They are also responsible for managing the total quality of the laboratory performance and are engaged in the continuous professional development and scientific research. They are educators and mentors involved in continuous education of medical biochemists, laboratory technicians and other health care professionals. The level of competence, knowledge and skills required for the specialist in medical biochemistry, seems to be equal to other EU countries (5,9,10).
Undergraduate and postgraduate education
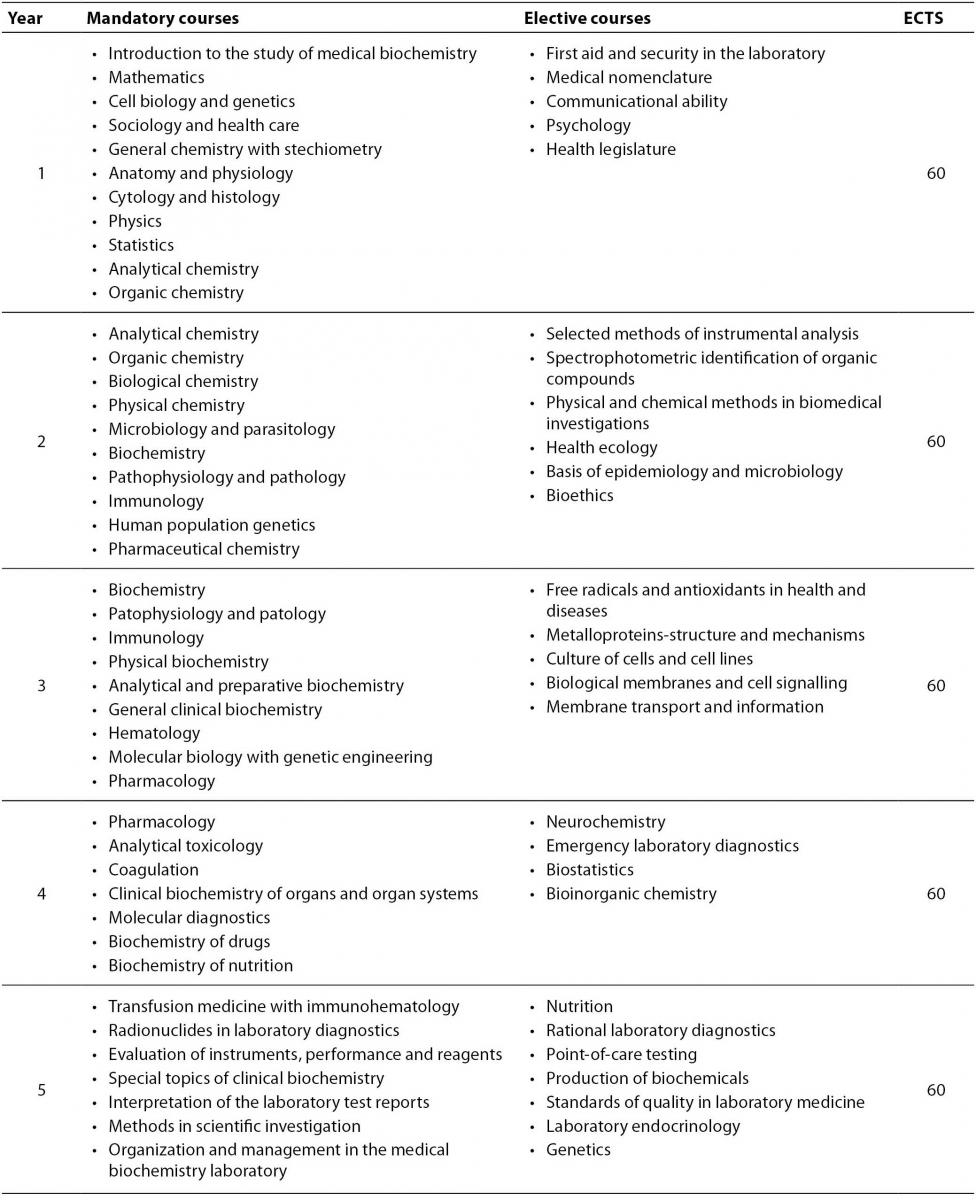
Medical Biochemistry has been studied for almost 50 years at the Faculty of Pharmacy and Biochemistry (11), whose curriculum was recently redesigned according to the requirements of the Bologna declaration (12). University degree (Master of Science) is earned after the 5 years of the studies. Obligatory and elective undergraduate courses in Medical Biochemistry at the Faculty of Pharmacy and Biochemistry are listed in Table 1. European Credit Transfer and Accumulation System (ECTS) credits are assigned to all subjects and are fully transmittable among other universities in Croatia as well as across Europe (13). Students are offered the opportunity to experience the laboratory practices from the first year of the curriculum. Practical part of the teaching is performed in several teaching hospital laboratories and in clinical departments. Emphasis is given to the problem based learning, clinical cases with discussions and self-oriented learning. Medical biochemists with a Master of Science degree can either proceed with one year training and specialization in medical biochemistry or (and) continue their education to get the PhD degree.
Table 1. Undergraduate study of Medical biochemistry at the Faculty of Pharmacy and Biochemistry in Croatia: list of mandatory and elective courses.
The Faculty of Pharmacy and Biochemistry provides the postgraduate education within the three years of Doctoral studies for a) Pharmacy sciences and b) Biomedical sciences. PhD degree is requirement for a head of the clinical laboratory at the university hospital. Also, only specialists with a PhD and Assistant professor degree are entitled to be members of the examination board for the exam at the end of the vocational training (mentioned below). However, PhD degree is not a requirement for medical biochemistry specialists to practice the profession in a laboratory.
Register of medical biochemists
Register of medical biochemists is kept by the Croatian Chamber of Medical Biochemists (CCMB), established in 1995. The Croatian Chamber of Medical Biochemists is an independent professional organization responsible for professional regulation, licensing and continuous education of its members. Since it was established, CCMB has issued numerous regulatory documents. These regulatory documents are comprehensive laboratory standards and/or regulations to provide laboratories a guide in maintaining a minimum mandatory quality. The most relevant regulatory documents are:
- Quality assurance and good laboratory practice (1995);
- Continuous education and examination: content, deadlines and protocol (1995);
- Register of CCMB members (1995);
- Code of ethics (1996);
- Type of laboratory analyses (2003);
- Rules on auditing medical biochemistry laboratories and medical biochemists (2004);
- Harmonization of laboratory results in general medical biochemistry (2004) (14);
- Practicing medical biochemistry in physicians’ office (2005);
- Practicing point-of-care analysis (2005);
Upon graduation, medical biochemists enter the one year program of supervised practical training in laboratory. This one year postgraduate training is in contrast to the four years in most other European countries. However, the content of the undergraduate degree is much more vocational in Croatia, in contrast to other countries, where the primary qualification may be monovalent, like biology, pharmacy etc. This one year program of supervised practical training can be done in any medical biochemistry laboratory headed by the medical biochemistry specialist. After completion of that training, a State exam has to be taken. This exam is organized by the Croatian Ministry of Health and is mandatory. After completing training and exam, medical biochemist becomes registered as a member of CCMB and gets the license for independent work. Medical biochemists with a license may practice the profession, but may not be involved and responsible for some specialized laboratory tests (immunology, molecular diagnostics, toxicology etc.). The scope of assays and procedures for which a specialist degree is required, is defined by CCMB.
License is valid for a period of six years. During that period credits are earned within the program of continuous education provided by CCMB. Credits can also be earned for the education provided by other professional bodies, by attending and/or giving lectures at seminars, conferences, courses and workshops. Also, credits may be earned for published papers and books/chapters, as well as for earning a degree (PhD) and for specialization in medical biochemistry. Specialists who are mentors to young PhD students and residents also get credits for that. Credits are designated by CCMB to various educational activities according to the well defined criteria. Members should provide evidence for their credits earned at the end of the licensing period, in order to be re-licensed. Licensing is mandatory and regulated by the Government (Law on the Health Care, 1993).
The licensing policy and procedure in Croatia appears to be in some respect quite unique relative to other European countries. Namely, while licensing occurs in Croatia after one year of post-graduate practical training and an exam, in all European Union member states the minimum of training before registration is four years.
In majority of European countries professional registration is now required for practicing clinical chemistry profession (9) and registers are governed by various governmental bodies (Ministry of Health, Ministry of Education, Chambers etc.) and even professional societies (8). Also, a final examination is included in most countries. Specialists in clinical chemistry and laboratory medicine are in some countries obliged to continuous professional development whereas in others are not. In those countries where continuous professional development is obligatory, it may and may not be required for re-licensing, depending on the country.
What has also been regulated by the government is the responsibility of the CCMB to provide the quality control assurance system for medical biochemists and medical biochemistry laboratories. CCMB has organized the quality control assurance system into two separate modules: 1) audit for medical biochemists and medical biochemistry laboratories, and 2) national external quality control program. Both programs are mandatory.
Audits for medical biochemists and medical biochemistry laboratories are continuously supported by the governmental financial resources. CCMB has a committee for professional audit which aim is to continuously control and supervise the quality of medical biochemists and medical biochemistry laboratories. Auditors are trained by the Committee members, according to regulatory documents issued by the CCMB, based on the ISO 15189 standard for medical laboratories. Audits cover organizational, technical and professional aspects of the laboratory.
National external quality control program management is delegated to the Croatian Society of Medical Biochemists (CSMB). CSMB is an independent, professional association of all university degree laboratory professionals, whose membership is voluntary. CSMB provides the external analytical quality assessment scheme for the majority of general clinical chemistry, hematology and coagulation analyses (15). Participation for the external analytical quality assessment scheme for all laboratories in Croatia is mandatory. Although CSMB has recently launched a pilot extra-analytical survey, there is still not a formal and systematic program of quality assurance for the extra-analytical phase in our country. One recent survey study on extra-analytical phase in Croatia has indicated that there is a need for improving the extra-analytical practices, especially blood sampling procedures (16,17). Therefore, the formal EQA program covering the extra-analytical phase is highly recommended and should be implemented.
Vocational training
Vocational training for medical biochemists is regulated by the national regulatory document issued by the Ministry of Health. According to that document, only medical biochemists graduated from the Faculty of Pharmacy and Biochemistry are eligible for specialization in medical biochemistry. The duration of the entire period of vocational training is 48 months. Training program matches the European Syllabus (18) and it consists of postgraduate specialist study and four years of obligatory vocational training in designated hospitals. The content of the medical biochemistry postgraduate study comprises following courses: regulation of metabolism, clinical enzymology, molecular diagnostics, molecular pathobiochemistry, accreditation, organization and management of the medical biochemistry laboratory, analytical techniques in medical biochemistry laboratory, neurobiochemistry, autoimmune diseases, molecular-biological methods in medical-biochemical laboratory, toxicology and TDM, laboratory hematology, immunohematology and coagulation, endocrinology, transfusion medicine, tumour markers, biostatistics and method validation. The vocational training program for medical biochemistry comprises 15 months of medical biochemistry, 11 months of hematology and coagulation, 5 months of immunology, 2 months of microbiology and 11 months of point-of-care testing. The selection of the hospital that can host vocational trainees is done by the Ministry of Health.
Final examination after vocational training is obligatory and is under the responsibility of the Ministry of Health. The two-day examination comprises the practical part in the laboratory (day one) and a comprehensive oral exam (day two) in front of the three members of the examination board. Examination board members are medical biochemists with PhD degree, specialists in medical biochemistry, holding the minimum degree of Assistant professor, delegated by the Ministry of Health.
Accreditation standards
As in most of the European countries (19), laboratory accreditation is not mandatory in Croatia. So far there are only few medical biochemistry laboratories accredited according to ISO 15189 (20). Accreditation is recognized by the professional bodies as an inevitable step in future improvement of the quality of laboratory practices on the national level and CCMB is therefore continuously providing its membership with educational workshops and training courses on accreditation and laboratory management. Croatian Accreditation Agency (HAA) is an independent, non-commercial national accreditation body, entitled for accreditation of medical laboratories, which complies with all international and European standards for accreditation bodies recognized in the Republic of Croatia as the Croatian Standard HRN EN ISO/IEC 17011:2005. Technical assessors are specialists in medical biochemistry and are selected and trained by the national accreditation body (HAA). Whereas accreditation is playing an important role for contracting with health care insurance companies and reimbursement of expenses for health care in some European countries (18), accreditation status is not of relevance to health insurance agencies in Croatia.
Conclusion
There is a heterogeneity in definition of our profession as well as in the academic background of specialists in clinical chemistry and laboratory medicine across European countries as well as worldwide (21,22). Croatia is one good example of a highly regulated profession of medical biochemistry practitioners. Despite some obvious differences from other European countries, the profession has a similar interdisciplinary character and a comparable level of required competence and skills. The education lasts for 10 years (5 years of undergraduate study + 1 year of obligatory training + 4 years of vocational training) and is fully in accordance with the European standards. The official regulation of profession is established by law. There is a well established program for licensing and continuous education for medical biochemists, based on the credit points and strictly defined criteria.
One of the main goals on the national level is to promote high professional and scientific standards of the medical biochemistry profession to be fully prepared for the common platform for professionals in clinical chemistry and laboratory medicine at the European level.