Introduction
Chronic myeloid leukemia (CML) was discovered in the first half of the 19th century (1). This type of leukemia is characterized by abnormal granulopoiesis in bone marrow and peripheral blood. Important landmark of CML is Philadelphia chromosome (Ph chromosome), a result of a t(9;22) translocation, probably in a single hematopoietic stem cell. This type of gene rearrangement of breakpoint cluster region (BCR) on chromosome 22 to ABL kinase domain on chromosome 9 leads to development of a BCR-ABLfusion gene. The product of this fusion gene, BCR-ABL protein, has abnormal tyrosine kinase activity stimulating enlarged cell proliferation (2).
Historically, many revolutionary advances in therapy for chronic myeloid leukemia (CML) have been achieved over time. One of the most frequently used drugs for CML in the 1950s was busulfan, but it was shortly replaced with hydroxycarbamide. Interferon-alpha, introduced in therapy in the 1980s, was the first agent to induce any degree of Ph-chromosome negativity in bone marrow. Between 1980 and 2000, bone marrow transplantation was the first therapeutic option in young patients with HLA-matched donors. The most important therapeutic discovery was original ABL tyrosine kinase inhibitor, imatinib mesylate (IM). IM is a 2-phenylaminopyrimidine compound, a small molecule that effectively blocks the kinase activity of BCR-ABL protein. IM occupies the ATP-binding site of BCR-ABL protein, causing a closed configuration and inhibition of the enzyme activity of the protein (2). IM is administered orally with the initial dose of 400 mg per day and is the gold standard as first line therapy in newly diagnosed CML patients, because of its limited toxicity and high response rate (3).
The frequency of complete cytogenetic response (CCyR) is very high in IM-treated patients, which is evident from the fact that no Ph positive metaphase is found in the follow up sample. Once the patient has achieved CCyR, the most informative method to measure the level of BCR-ABL transcripts is real-time quantitative polymerase chain reaction (RQ-PCR) (1,4). In this methodology, the BCR-ABL transcript numbers are related to transcript numbers of a control gene (ABL) that is expressed in leukemia cells at approximately the same level as in normal cells. The result is generally expressed as log reduction in the BCR-ABL/ABL ratio, and achievement of 3 or more log reduction in BCR-ABL/ABL ratio in 18 months is called major molecular response (MMoR) (2,4). It has been shown that accurate measurement of BCR-ABL transcript reduction correlates inversely with the probability of disease progression (1). Furthermore, patients in MMoR can remain positive and stable, or can have undetectable BCR-ABL transcript during follow up. For non-responders, or those who never achieve MMoR, monitoring of the BCR-ABL/ABL ratio is crucial to revise therapeutic strategy. A high proportion of these patients develop resistance to the drug caused by ABL kinase domain mutations (5), or show undefined individual variations in pharmacokinetics. In these patients, optional treatment is increment in IM dose (600 or 800 mg/day), second generation tyrosine kinase inhibitors (nilotinib, dasatinib), or bone marrow transplantation (2).
The aim of this study was to evaluate the efficacy of a treatment protocol and to achieve better assessment of the individual patient response to treatment with IM by using sensitive quantitative PCR measurement of the BCR-ABL/ABL ratio, in order to stratify patients according to the risk of relapse, and to individualize treatment protocol.
Materials and methods
The study included 31 patients (13 women and 18 men) diagnosed with CML. Ten of these patients were pretreated with busulfan or interferon-alpha, and the remaining 21 patients were newly diagnosed CML patients. Patients were diagnosed and treated at Hematology Department, University Department of Medicine, Zagreb University Hospital Center. Initial IM dose for all patients was 400 mg/day. Patients were followed up for 39 (3–81) months; median (range).
RNA was isolated from bone marrow or peripheral blood cells by using QuickPrep mRNA purification kit (GE Healthcare, UK) according to the manufacturer’s instructions.
RQ-PCR was performed according to EAC protocol using TaqMan technology (LightCycler, Roche) (6). RNA was reverse transcribed in reaction volume of 20 μL using FirstStrand cDNA synthesis kit (Amersham Biosciences, UK). Amplification of fusion BCR-ABL and ABL as a control gene (RQ-PCR) was performed using FusionQuant Kit for RQ-PCR analysis of BCR-ABL Mbcr (Ipsogen, France) according to the manufacturer’s instructions. All samples were analyzed in triplicates for both BCR-ABL and ABL, and the BCR-ABL/ABL ratio was calculated (7).
Standardized baseline was derived from the BCR-ABL/ABL ratio obtained from untreated patients at diagnosis and was used for calculation of log reduction for all follow up samples (1,6).
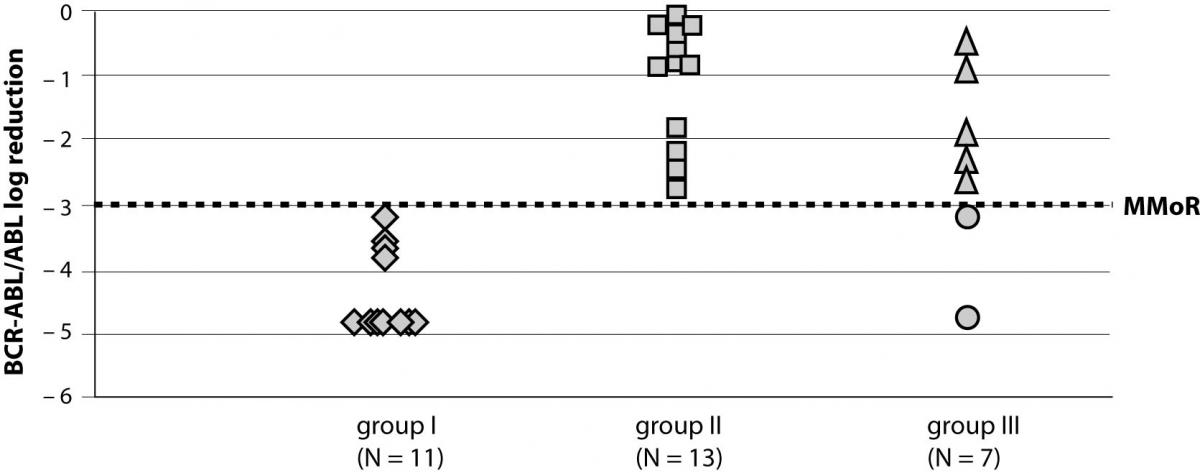
Patients were divided into groups according to calculated log reduction of the BCR-ABL/ABL ratio during 18-month follow up of IM therapy. Group I consisted of 11 patients that achieved more than 3 log reduction in 18 months; group II included 13 patients that had less than 3 log reduction in 18 months of IM therapy; and group III included 7 patients with a follow up of less than 18 months.
Results
A total of 31 patients diagnosed with CML that started using therapy with IM (400 mg/day) were followed-up for a median time of 39 (range 3–81) months. During the follow up period, six of 31 patients reached an undetectable BCR-ABL transcript by RQ-PCR. Results on the response rate to IM therapy at 18 months for groups I and II and in the last follow up sample for group III are presented in Figure 1.
Figure 1. BCR-ABL/ABL log reduction in study groups achieved at 18 months (groups I and II) and in the follow up sample (group III). MMoR – major molecular response
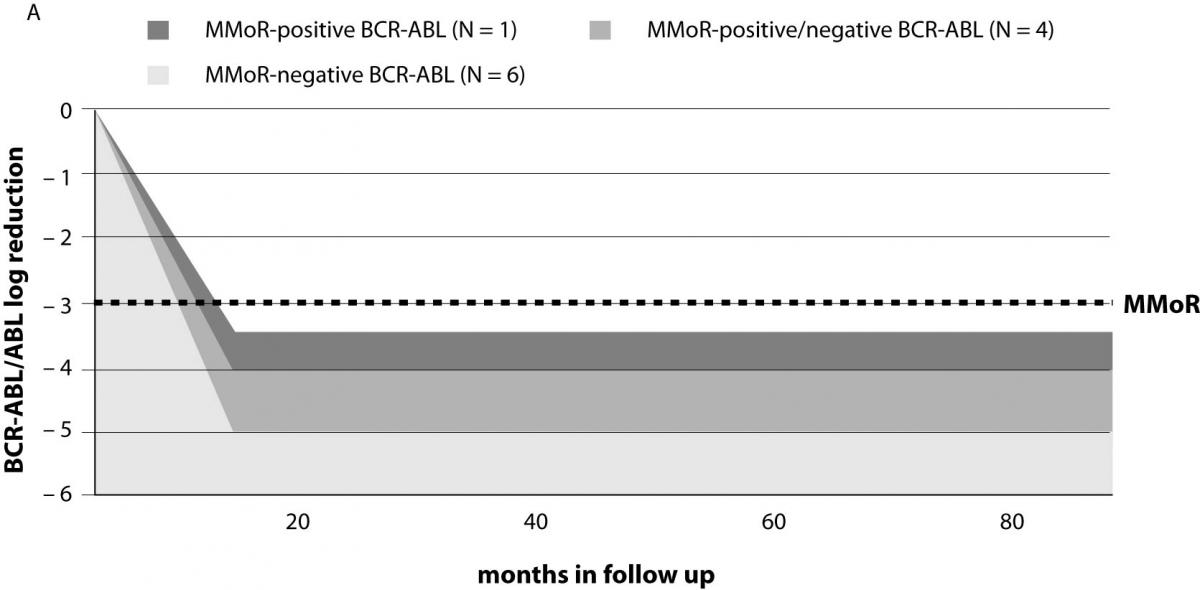
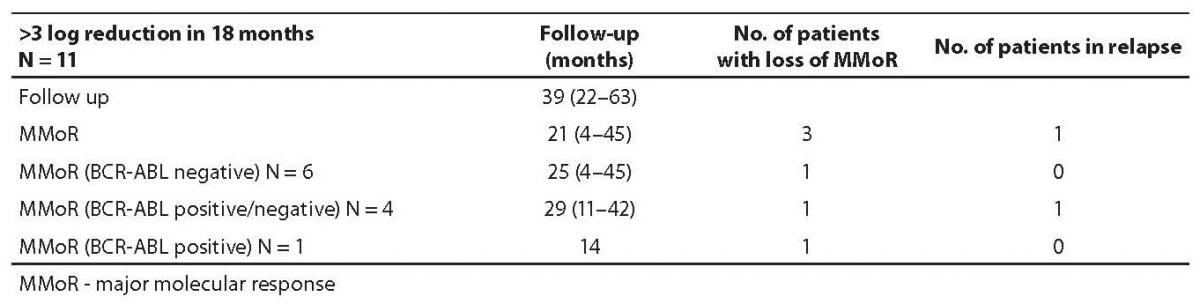
Results on 11 patients (group I) that achieved 3 or more log reduction and met the criteria for MMoR are presented in Table 1. Schematic presentation of IM therapy monitoring and MMoR achievement in this group is shown in Figure 2a. These patients were monitored for a median time of 39 (range 22–63) months, and remained in MMoR for 21 (range 4–45) months. Six of these patients, including three patients pretreated with busulfan and interferon-alpha, having achieved MMoR, maintained undetectable BCR-ABL transcript for a median time of 25 (range 4–45) months. None of these patients relapsed. Another four patients had time points with detectable BCR-ABL transcript during the monitoring period (median time) of 29 (range 11–42) months. One of them lost MMoR and relapsed after 11-month follow up. Only one patient achieved MMoR with detectable BCR-ABL transcript during 14 months and at one time point lost response but never relapsed.
Table 1. Follow up results in group I
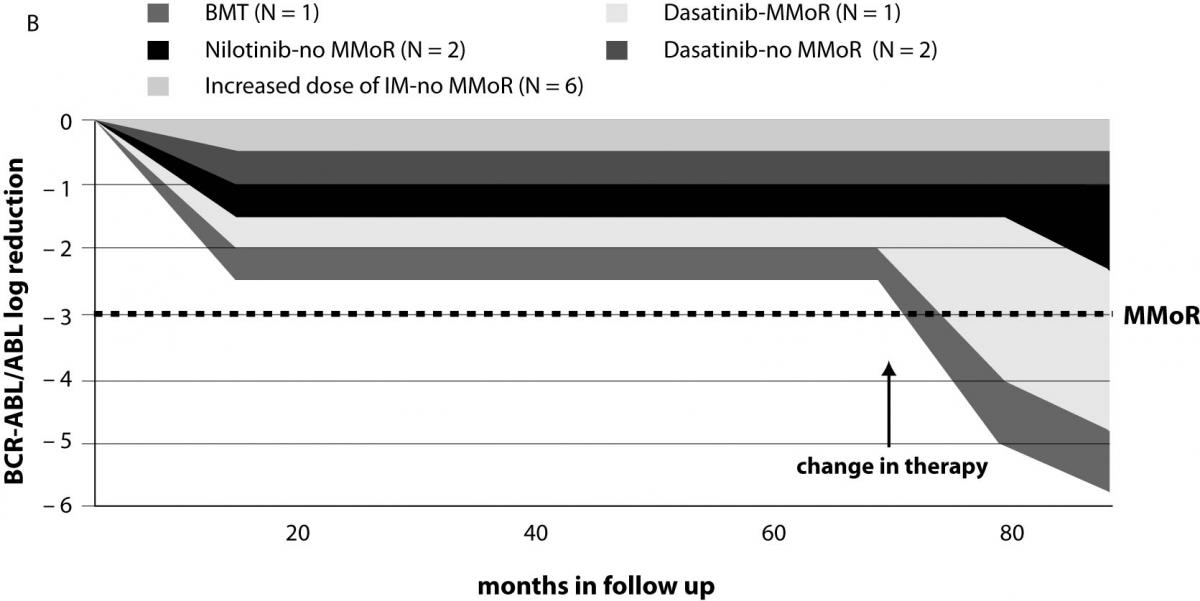
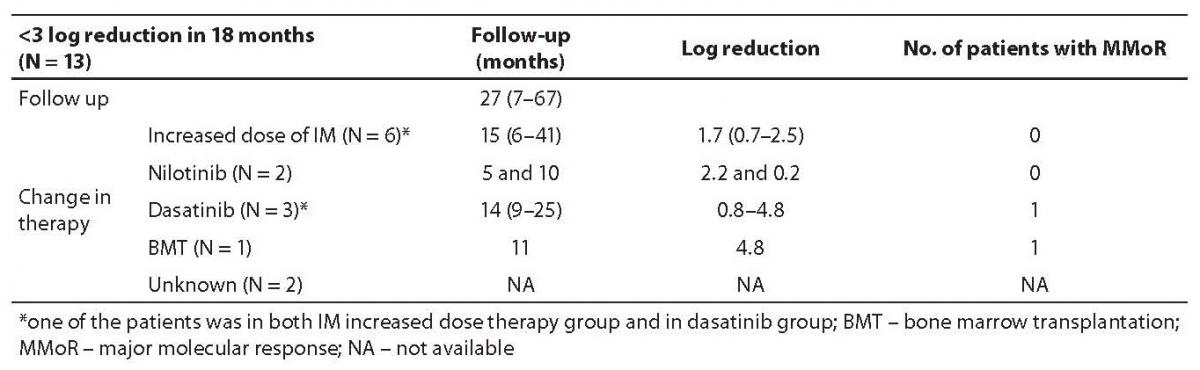
Results on 13 patients (group II) that did not achieve MMoR in 18 months of IM therapy are presented in Table 2. Schematic presentation of IM therapy monitoring without achievement of MMoR in group II is shown in Figure 2b. In these patients, therapeutic modification occurred after 27 months of follow up (range 7–67): 6 patients started with an increased IM dose (600 or 800 mg/day). In the monitoring period of 15 months (range 6–41), none of them achieved MMoR. One of these patients started dasatinib after failure of increased IM therapy dose (monitoring time 41 months) and in 9 months achieved more than 3 log reduction. Another two patients on dasatinib therapy achieved less than one log reduction in 14 and 25 months of follow up. Two other patients started nilotinib therapy; one achieved only 0.2 log reduction in 10 months and the other 2.2 log reduction in 5 months. The patient without any response to IM therapy underwent bone marrow transplantation with undetectable BCR-ABL transcript 11 months later. Seven patients from this group were pretreated with busulfan and interferon-alpha. Five of them as well as two of the six newly diagnosed CML patients did not respond to therapy modification. For two pretreated patients in this group, there are no data on the course of therapy.
Figure 2. Monitoring patients on IM therapy: (a) achievement of major molecular response (MMoR) in group I; (b) MMoR was never achieved in group II. BMT – bone marrow transplantation; MMoR – major molecular response
Table 2. Follow up results in group II
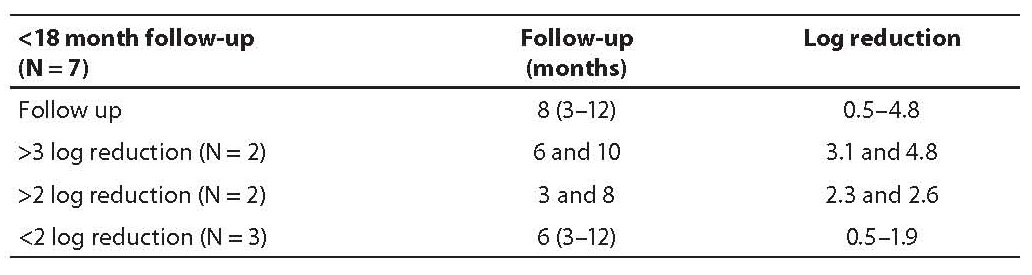
Group III included seven newly diagnosed CML patients (Table 3) monitored for less than 18 months (8 months, range 3–12 months) and therefore MMoR could not be estimated. Two of them achieved 3 log reduction at 6 and 10 months of follow up, the other two achieved 2 log reduction in 3 and 8 months. The last three patients achieved less than 2 log reduction in the median time of 6 (range 3–12) months.
Table 3. Follow up results in group III
Discussion
Measurement is fundamental in hematology, especially in case of leukemia where counting and identifying cells in blood and bone marrow are traditional (8). For many years, response to CML therapy was measured with a number of leukemic cells originating from abnormal granulopoiesis. With time, improvement in therapy required ever more sensitive techniques for better assessment of therapeutic response (9). In the 1960s, specific CML chromosome abnormality, known as Ph chromosome, was discovered. Identification and quantification of Ph positive metaphases have become valuable tools to confirm the diagnosis and to monitor therapeutic response (1,2). However, desire for further improvement in sensitivity has led to the introduction of molecular techniques for measuring BCR-ABL transcripts. Discovery of the ABL tyrosine kinase inhibitor, imatinib mesylate, was revolutionary in the treatment of CML and the only method to measure the level of remission accomplished is real time quantitative polymerase chain reaction (2,7,11). Measuring the level of BCR-ABL transcript, presented as log reduction in the BCR-ABL/ABL ratio, is used to enable better assessment of response of individual patients to treatment with IM and to evaluate the efficacy of a treatment protocol. It can also be used to stratify patients according to the risk of relapse and is crucial to revise therapeutic strategy (1,4).
Although complete estimate of therapeutic response according to the European Leukemia Net includes clinical assessment, blood count, cytogenetic revision of bone marrow and calculation of the BCR-ABL/ABL ratio every 3 months from therapy initiation (2), in this study only log reduction in the BCR-ABL/ABL ratio was used to monitor CML patients on the standard treatment protocol with IM. In general, reduction in the BCR-ABL/ABL ratio correlates closely with the increasing hematological as well as cytogenetic response. It is known that 2 log reduction in the BCR-ABL/ABL ratio in 12-month follow up is in concordance with Ph negativity in bone marrow (CCyR) (10). In this period, RQ-PCR becomes the only tool for further monitoring of molecular response, which depends on the rate and degree of BCR-ABL transcript reduction (2,11).
Patients in study group I achieved MMoR with detectable or undetectable BCR-ABL transcript during 2-year follow up and fulfilled the criteria for favorable long term prognosis according to literature data (10). Despite this, monitoring should be performed indefinitely, including patients with undetectable BCR-ABL transcript because of limited sensitivity of RQ-PCR which differs with complete disappearance of leukemia (2,11).Results in study group I indicated that even those patients could lose MMoR and relapsed due to acquired resistance to IM or to interactions with other drugs. One of the patients lost MMoR when he started using, what seemed to be, a competitive drug (tamsulosin hydrochloride). Incidentally, when this patient stopped using the same drug, BCR-ABL transcript became undetectable again. A hypothetical mechanism could be competition with IM for a transporter and consequently availability of IM in the cell. The other patient with MMoR and detectable BCR-ABL transcript relapsed after 11 months, a reason more why these patients should be more carefully monitored to predict the course of disease and possible relapse.
Group II patients never achieved MMoR with IM. Most of them were diagnosed with CML years before IM era and were pretreated with busulfan and interferon-alpha for different periods of time, and therefore they were not representative for evaluation of treatment efficacy. A small minority of newly diagnosed CML patients from this group that did not achieve MMoR with IM required different therapy approach according to literature, i.e. a higher dose of IM and other tyrosine kinase inhibitors (10). Our results indicated the increased dose of IM to provoke temporary response in a minority of patients but none of them achieved MMoR (Table 2). On the other hand, the use of a second generation ABL tyrosine kinase inhibitors, dasatinib or nilotinib, which depends on a point mutation found in an IM-resistant patient, produces high response rates, with the exception of T315I mutant clone (2). The results obtained in two patients that achieved MMoR in less than a year from therapy introduction, one with dasatinib and the other with nilotinib, confirmed the efficacy of these drugs. Additional studies are stimulated to test long term efficacy of these drugs and possible adverse events (3). That bone marrow transplantation remains first choice therapy for young IM-resistant patients with HLA-matched donor was confirmed by a patient from study group II. This patient did not respond to IM therapy but after transplantation maintained MMoR for almost a year.
If the same analogy from the last two groups is applied, further development could be predicted in group III patients, i.e. seven newly diagnosed CML patients with IM as first line therapy. Two of these patients reached more than 3 log reduction in 6 and 10 months, and the other two achieved more than 2 log reduction in 3 and 8 months. It is reasonable to expect for these patients to achieve and maintain MMoR. The last three patients had less than 2 log reduction in 6 months but it is still too early to predict failure despite their poor response to IM therapy.
In conclusion, the success of IM therapy for CML has led to recommendations based on published studies (1,2,6,7). Patients diagnosed with CML should receive initial treatment with IM (starting dose 400 mg per day) and, if they respond, should continue with therapy indefinitely. All patients should be monitored at defined time points, including clinical assessment, blood count, bone marrow cytogenetics, and BCR-ABL transcript measurement every 3 months to determine non-responders and IM resistance at each time point of the follow up. Patients who fail treatment with IM or lose response should be screened for the presence of ABL kinase mutations in order to ensure proper change in therapy. The use of second generation tyrosine kinase inhibitors depends on a point mutation discovered in ABL gene and is not recommended for patients with a T315I mutant clone. These patients have a possibility for bone marrow transplantation or use of standard chemotherapy (hydroxycarbamide, interferon-alpha or cytarabine).
The results obtained in this study confirmed that compliance with these recommendations ensures optimal treatment for patients with CML.
Notes
Potential conflict of interest
None declared.
References
1. Hughes T, Deininger M, Hochhaus A, Branford S, Radich J, Kaeda J, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood 2006;108:28-37.
2. Goldman JM. How I treat chronic myeloid leukemia in the imatinib era. Blood 2007;110: 2828-37.
3. Kantarjian HM, Cortes J, Guilhot F, Hochhaus A, Baccarani M, Lokey L. Diagnosis and management of chronic myeloid leukemia: a survey of American and European practice patterns. Cancer 2007;109:1365-75.
4. Baccarani M, Saglio G, Goldman J, Hochhaus A, Simonsson B, Appelbaum F, et al. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European Leukemia Net. Blood 2006;108:1809-20.
5. De Keersmaecker K, Cools J. Chronic myeloproliferative disorders: a tyrosine kinase tale. Leukemia 2006;20:200-5.
6. Gabert J, Beillard E, van der Velden VH, Bi W, Grimwade D, Pallisgaard N, et al. Standardization and quality control studies of ‘real-time’ quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia - a Europe Against Cancer program. Leukemia 2003;17:2318-57.
7. Branford S, Cross NC, Hochhaus A, Radich J, Saglio G, Kaeda J, et al. Rationale for the recommendations for harmonizing current methodology for detecting BCR-ABL transcripts in patients with chronic myeloid leukaemia. Leukemia 2006;20:1925-30.
8. Morley A. Quantifying leukemia. N Engl J Med 1998;339:627-9.
9. Cross NC. Minimal residual disease in chronic myeloid leukaemia. Hematol Cell Ther 1998;40:224-8.
10. Hochhaus A, Druker B, Sawyers C, Guilhot F, Schiffer CA, Cortes J, et al. Favorable long-term follow-up results over 6 years for response, survival, and safety with imatinib mesylate therapy in chronic-phase chronic myeloid leukemia after failure of interferon-alpha treatment. Blood 2008;111:1039-43.