Introduction
The modern era in the psychopharmacological treatment of schizophrenia started with the observation that chlorpromazine, originally studied for its sedative effects, had the ability to treat delusions and hallucinations (1). Later, due to the hypothesis that suggested that the antipsychotic action of chlorpromazine was caused by its ability to block the stimulation of brain dopamine receptors, many other substances like butyrophenes or phenothiazines were synthesized for that purpose (2,3). Not only did that group of antipsychotic drugs, resulting from the dopamine hypothesis of schizophrenia, shape the treatment of schizophrenia but also the basic concept of the process of the disease (4). Further investigations showed that productive symptoms of schizophrenia are not only of interest for the patients’ rehabilitation, but cognitive symptoms of the schizophrenic disease process are also recognized as important components for many patients (5,6). These concepts bring new light on the etiology as well as psychopharmacology of schizophrenia; the dopaminergic system is not more important than other symptoms, especially serotonergic system in the pathophysiology of schizophrenia (7,8). This concept provides new psychopharmacologic strategies and today’s new antipsychotics, also called atypical, are the first line of drugs for the treatment of schizophrenia (9).
However, current classification divides the antipsychotics only in typical, dopaminergic antagonists and atypical antipsychotics with action on multiple neurotransmitter systems, mostly dopaminergic and serotonergic (10). The question is whether the atypical antipsychotics are indeed pharmacologically similar, or there exist some substantial differences among them. Cardinal differences between atypical antipsychotics and typical antipsychotics are in the range of side effects, considering that typical antipsychotics can produce extrapyramidal symptoms as well as elevated prolactin (PRL) levels (2,11,12). The elevation of blood PRL levels in patients treated with typical antipsychotics is a consequence of their antagonistic dopaminergic activity, since dopamine is a strong PRL inhibitory factor (13). Hyperprolactinemia in patients with schizophrenia has been linked to various health issues such as sexual dysfunction and infertility (14), galactorrhea, menstrual irregularities (15), bone mineral density loss leading to osteopenia and osteoporosis (16). There has also been recognition that hyperprolactinemia may be associated with metabolic disturbances in patients with schizophrenia (17).
The aim of our study was to investigate the prolactin (PRL) response after specific dopaminergic probe with sulpiride as well as serotonergic probe with clomipramine in patients with schizophrenia treated with clozapine, olanzapine, risperidone, and haloperidol.
Subjects and methods
Subjects
We prospectively recruited a consecutive series of 124 male patients with schizophrenia admitted to the Department of Psychiatry, Sestre milosrdnice University Hospital, Zagreb, Croatia, from January 2007 to September 2007. We decided to selectively include only male patients, because they have lower and constant blood PRL concentrations in comparison to female patients.
Inclusion criteria were: patients who had only one antipsychotic in their therapy and had good therapeutic response to that drug, did not take any other drugs except for benzodiazepine and that they did not have any other psychiatric or medical problem besides schizophrenia. Forty-four male patients with a mean age of 34 ± 6 years fulfilled the inclusion criteria and were included in the study. All patients fulfilled DSM IV criteria for schizophrenia (18). There were 34 patients with a paranoid-hallucinatory, 5 with a disorganized, 1 with a catatonic and 4 with an undifferentiated form of schizophrenia. Mean duration of illness was 9 ± 4 years. Patients were divided into the following study subgroups according to their antipsychotic therapy: haloperidol (N = 11), clozapine (N = 12), risperidone (N = 11) and olanzapine (N = 10). The haloperidol dose was 15 mg, the clozapine dose 300 mg, the risperidone dose 6 mg, and the olanzapine 20 mg per day for each patient. These doses were equal to chlorpromazine equivalents. The duration of that psychopharmacotherapy was less than five weeks prior to this experiment.
The ethical committee of the Sestre milosrdnice University Hospital approved this research and all patients gave informed consent in order to participate in the study.
Endocrine measurement
At 8 a.m., after an overnight fast, an indwelling venous catheter was inserted into a forearm vein, maintaining normal saline infusion. After a 15-minute-rest subjects were administered 100 mg of sulpiride intramuscularly. Blood samples for PRL concentration were drawn at 0, 30, 120 and 180 minutes after the infusion.
Seven days after, the same procedure was repeated with clomipramine in doses of 75 milligrams intramuscularly, and blood samples were collected at 0, 30, 60, 120, 180, and 240 minutes after clomipramine injection. All stimulation tests were performed according to standardized protocols previously described in literature (19).
All blood samples were centrifuged for 15 minutes at 2500 rpm. Serum was aliquoted and stored at –20 ºC until analysis. PRL concentration was determined by fluoroimmunoassay (FIA) using the Delfia prolactin commercial kit (Wallac Oy, Turku, Finland). All tests were run in duplicate. As declared by the manufacturer, assay sensitivity, intra-assay coefficient of variation and inter-assay coefficient of variation were 0.04 ng/mL, 2.6% and 3.4%, respectively.
DPRL was calculated at the maximum rise in PRL level after an injection of sulpiride after 30 minutes, or clomipramine after 180 minutes, minus basal PRL level. Our laboratory PRL reference interval for male patients is 2.3–11.5 ng/mL.
Statistical analysis
Data were expressed as mean ± SD. The discrepancies between data distribution and normal distribution were examined by the Kolmogorov-Smirnov test for each group. Comparisons of basal and DPRL between haloperidol, clozapine, risperidone, and olanzapine groups were evaluated by the analysis of variance (ANOVA). We used the post-hoc Scheffé method for pair-wise comparisons. Statistical significance was set at the level of 0.01. All dana analyses were conducted using the statistical package SPSS 8.0 (SPSS for Windows 8.0, SPSS., Chicago, IL, USA).
Results
Basal PRL levels
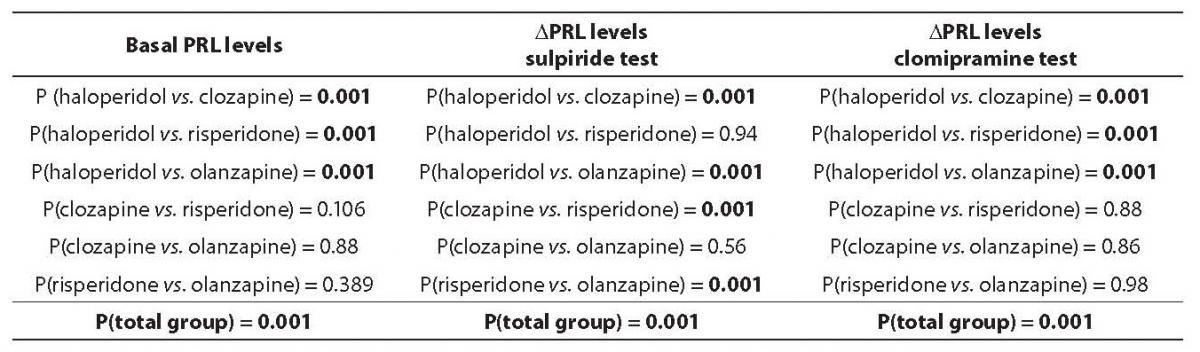
Mean basal PRL values were 32.0 ± 7.8 ng/mL in haloperidol group, 4.7 ± 1.1 ng/mL in clozapine group, 10.1 ± 1.6 ng/mL in risperidone group, and 6.4 ± 2.4 ng/mL in olanzapine group. The observed difference in mean basal PRL levels between patient subgroups was statistically significant (P < 0.001). Post-hoc Scheffé test revealed that the difference was significant between haloperidol group vs. all three other subgroups (clozapine, olanzapine, and risperidone) (Table 1.). Clozapine, risperidone and olanzapine subgroups did not differ among each other. The mean PRL concentration in the haloperidol subgroup was above the reference interval, whereas clozapine, olanzapine and risperidone subgroups had mean PRL concentration within the reference interval.
PRL in dopaminergic probe
Mean DPRL values after the sulpiride injection were: 8.2 ± 1.7 ng/mL in haloperidol, 94.3 ± 8.7 ng/mL in clozapine, 5.3 ± 1.3 ng/mL in risperidone and 46.6 ± 16.0 ng/mL in olanzapine patient subgruop. The observed difference in mean DPRL levels after sulpiride injection was statistically significant among patient subgroups (P < 0.001). Post-hoc Scheffé test showed that mean DPRL levels after sulpiride injection were substantially higher in clozapine and olanzapine subgroups compared to haloperidol and risperidone groups. There was no statistically significant difference between haloperidol and risperidone groups in DPRL value after sulpiride injection (Table 1).
Table 1. Analysis of variance (ANOVA), and Post-hoc Scheffé test in the analysis of difference in prolactin (PRL) basal levels, and after sulpiride and clomipramine probe
PRL in serotonergic probe
Mean DPRL values after the clomipramine injection were: 12.7 ± 4.7 ng/mL in haloperidol, 0.8 ± 0.3 ng/mL in clozapine, 1.5 ± 0.7 ng/mL in risperidone and 1.9 ± 1.1 ng/mL in olanzapine subgroup. The observed difference in mean DPRL levels after serotonergic probe with clomipramine injection was statistically significant between patient subgroups (P < 0.001). Post-hoc Scheffé test revealed that mean DPRL levels after clomipramine injection were substantially higher in haloperidol compared to clozapine, risperidone and olanzapine subgroups. The mean DPRL values after a serotonergic probe with clomipramine did not differ among clozapine, risperidone, and olanzapine subgroups (Table 1).
Discussion
Our results suggest that there is a difference in pharmacological characteristics of atypical antipsychotics risperidone, olanzapine, and clozapine. We used the sulpiride test in our neuroendocrine probe to investigate the dopaminergic activity because of its strong antagonist dopaminergic receptor type 2 (D2) activity, and good PRL release after the sulpiride application (20). The second test we performed was the clomipramine test. We chose clomipramine because of its strong selective serotonin inhibitor (21), and because clomipramine in other studies showed a favorable effect on PRL release (22).
Patients treated with typical antipsychotics often have elevated PRL levels because of their strong antagonistic activity on dopaminergic receptors (23). In our assessment, patients with schizophrenia treated with haloperidol also showed elevated basal PRL levels. Patients with schizophrenia treated with atypical antipsychotics, clozapine and olanzapine, had basal PRL levels within a normal range, while in the risperidone group the basal levels of PRL were slightly elevated or upper reference range values. The potential of risperidone for serotonergic activity equals that of clozapine and olanzapine, while in the dopaminergic activity its potential is similar to that of a typical antipsychotic, haloperidol. These findings put risperidone (as an atypical antipsychotic) in a new, borderline position between true typical and true atypical antipsychotics, and they also shed a new light on the classification of antipsychotics.
In other studies, risperidone is classified as an atypical antipsychotic because of its strong serotonergic activity (10). But when we analyzed the clinical effect of risperidone in comparison to other atypical antipsychotics, especially clozapine, risperidone’s activity on the symptoms of schizophrenia could be marked more as “typical” then “atypical”. On the other hand, while analyzing the clinical effects of risperidone compared to typical antipsychotics, risperidone had better effects on negative symptoms,which is a characteristic of atypical antipsychotics rather than the typical ones (24).
Risperidone’s range of side effects is more similar to those of typical antipsychotics, especially regarding extrapiramidal symptoms and the rise of PRL level (25). Thus, other authors as well as our investigation pointed out that it is necessary in the future to reformulate the concept of antipsychotics, especially regarding what is “typical” or “atypical”.
With respect to history data, antipsychotic activity introduces new conceptualization of neurotransmitter systems involved in pathology of schizophrenia (26). In reduction of side effects, improvement of negative symptoms, and therapeutic effect of treatment, resistant schizophrenia was a point in the development of atypical antipsychotics (27). In this concept, the development of new antipsychotics with dual dopaminergic and serotonergic activity was influenced by a new hypothesis of serotonergic/dopaminergic disbalance in schizophrenia (28).
Nowadays the literature suggests that many neurotransmitters are involved in the neurochemistry of schizophrenia other than serotonin and dopamine. For example,researchers especially pointed out NMDA, GABA and other systems (29). However, in the future we can expect new drugs which would act therapeutically through other systems rather than dopamine or serotonin ones (30,31). We think there is a need for analyzing the effects of antipsychotic drugs on the clinical picture, the course, and outcome of schizophrenic symptoms, as well as for assessing the antipsychotic action on neurotransmitters.
Our study had several limitations. Firstly, our study groups were too small. In further studies we need to increase the number of patients in the groups and expand research on some other antipsychotics, i.e. quetiapine, and aripiprazole. Also, we did not compare prolactin response and clinical severity of schizophrenia symptoms controlled by the psychiatric scale such as PANSS.
In conclusion, there is a difference in PRL response after dopaminergic and serotonergic probe in patients with schizophrenia treated with clozapine, olanzapine, risperidone, and haloperidol. This different response in neuroendocrine model is due to neurotransmitter systems being occupied by different antipsychotic drugs. Further analyses with other atypical antipsychotics are necessary to revise current classification of antipsychotics and possibly introduce a new classification of these drugs.
Acknowledgement
This study was supported by Ministry of Science, Education and Sports, Republic of Croatia, project #134-0000000-3372.
Notes
Potential conflict of interest
None declared.
References
1. Lehman HE, Hanrahan GE. Chlorpromazine: new inhibiting agent for psychomotor excitement and manic states. Arch Neuro and Psychiatry 1994;71:221-37.
2. Casey De. Neuroleptic drug-induced extrapiramidal syndromes and tardive dyskinesia. Schizophr Res 1991;10:21-8.
3. Goldeberg SE, Klerman GL, Cole JO. Changes in schizophrenia psychopathology and ward behaviour as a function of phenothiazine treatment. Br J Psychatry 1965;111:120-33.
4. Seeman P, Niznik HB. Dopamine receptors and transporters in Parkinson’s disease and schizophrenia. FASEB J 1990;4:2737-44.
5. Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry 1996;153:321-30.
6. Mernik K, Linaker OM. Factors that influence self-reported compliance for neuroloeptics. E J Psychiatry 2001;1:5-12.
7. Carlsson A, Hansson LO, Waters N, Carlsson ML. Neurotransmitter aberrations in schizophrenia: New perspectives and therapeutic implications. Life Sciences 1997;2:75-94.
8. Leysen JE, Jansen PMF, Heylen L, Gommeren W, Van Gompel P, Lesage As. Receptor interactions of new antipsychotics: Relation to pharmacodynamic and clinical effects. Int J of Psy in Clin Prac 1998;2 Suppl 1:3-17.
9. Wirshing WC, Marder SR. Efficacy and dosing issues of novel antipsychotics. Int J Psychiatry in Clinical Practice 1998;2 Suppl 1:35-8.
10. Kapur S, Zipurskiy RB, Remington G. Clinical and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone, and dopamine in schizophrenia. Am J Psychiatry 1999;156:286-93.
11. Guldensky GA, Porter JC. Release of dopamine from tuberoinfundibilar neurons into pituitary stalk blood following prolactin or haloperidol administration. Endocrinology 1980;106:525-9.
12. Tran PV, Dellva MA, Tollefson GD, Beasley CM, Potvin JH, Kiesler GM. Extrapiramidal symptoms and tolerability of olanzapine versus haloperidol in the acute treatment of schizophrenia. J Clin Psychiatry 1997;58:205-11.
13. Fuente JR, Rosenbaum AH. Prolactin in psychiatry. Am J Psychiatry 1981;138:1154-60.
14. Bitter I, Basson B, Dossenbach M. Antipsychotics treatment and sexual disfunctioning in first-time neuroleptic-treated schizophrenic patients. Int Clin Psychopharmacology 2005;15:111-7.
15. Windgassen K, Wesselman U, Schulze Monking H. Galactorrhea and hyperprolactinemia in schizophrenic patients on neuroleptics: frequency and aetiology. Neuropsychobiology 1996;33:142-6.
16. Hummer M, Malik P, Gasser R, Hofer A, Kemmler G, Nevada R, et al. Osteoporosis in patients with schizophrenia. Am J Psychiatry 2005;162:162-7.
17. Kovak-Mufić A, Karlović D, Martinac M, Marčinko D, Letinić K, Vidrih B. Metabolic side-effects of novel antipsychotics drugs. Biochemia Medica 2007;17:178-87.
18. American Psychiatric Association. Diagnostic and statistical manual of mental disorders IV, DSM IV. APA, Washington, 1994.
19. Wolkowitz OM, Rothschild AJ. Psychoneuroendocrinology. The Scientific Basis of Clinical Practice. APA, Washington, 2003.
20. Nathan RS, Van Kamen DP. Neuroendocrine effects of antipsychotic drugs. In: Burrovs GD, Norman TR, Davies B, eds. Antipsychotics: Drugs in psychiatry, Vol 3. New York, Elsevier, 1985.
21. Keltner Nl, Foulks DG. Psychotropic drugs. St. Louis, Mosby, 1998.
22. Hall H, Orgen SO. Effects of antidepressant drugs on different receptors in the brain. E J Pharmacology 1991;70:393-407.
23. Markinos M, Hatzimandis J, Lykouros l. Neuroendocrine serotoninergic and dopaminergic responsivity in male schizophrenic patients during treatment with neuroleptics and after switch to risperidone. Psychopharmacology 2001;157:55-9.
24. Kasper S. Risperidone and olanzapine: optimal dosing for efficacy and tolerability in patients with schizophrenia. Int Clin Psychopharmacology 1998;13:253-62.
25. Kleinberg DL, Brecher M, Davis JM. Prolactine levels and adverse events in patients treated with risperidone. J Clin Psychopharmacology 1998;18:308-15.
26. Davis KL, Kahn RS, Ko G. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry 1991;148:1474-86.
27. Reus VI. Olanzapine: a novel atypical neuroleptic agent. Lancet 1997;349:1264-5.
28. Mooren R, Verhoven WMA, Tuinier S, Van de Berg YWMM, Fekkes D, Pepplinkhuizen L. Olanzapine in relapsing schizophrenia. Efficacy and serotonergic parameters. Eur J Psychiat 2001;2:91-100.
29. Meltzer HY. Treatment of schizophrenia and spectrum disorders: pharmacotherapy, psychosocial treatments, and neurotransmitter interactions. Biol Psychiatry 1999;46:1321-7.
30. Kapur S, Seeman P. Does fast dissociation from the dopamine receptor explain the action of atypical antipsychotic? A new hypothesis. Am J Psychiatry 2001;158:360-9.
31. Meltzer HY. The role of serotonin in antipsychotic drug action. Neuropsychopharmacology 1999;21:106-15.