References
1. Jaffe AS. The clinical impact of the universal diagnosis of myocardial infarction. Clin Chem Lab Med 2008;46:1485-8.
2. Lippi G, Targher G, Franchini M, Plebani M. Genetic and biochemical heterogeneity of cardiac troponins: clinical and laboratory implications. Clin Chem Lab Med 2009;47:1183-94.
3. Plebani M, Zaninotto M. Cardiac troponins: what we knew, what we know - where are we now? Clin Chem Lab Med 2009;47:1165-6.
4. Panteghini M. A critical appraisal of experimental factors influencing the definition of the 99th percentile limit for cardiac troponins. Clin Chem Lab Med 2009;47:1179-82.
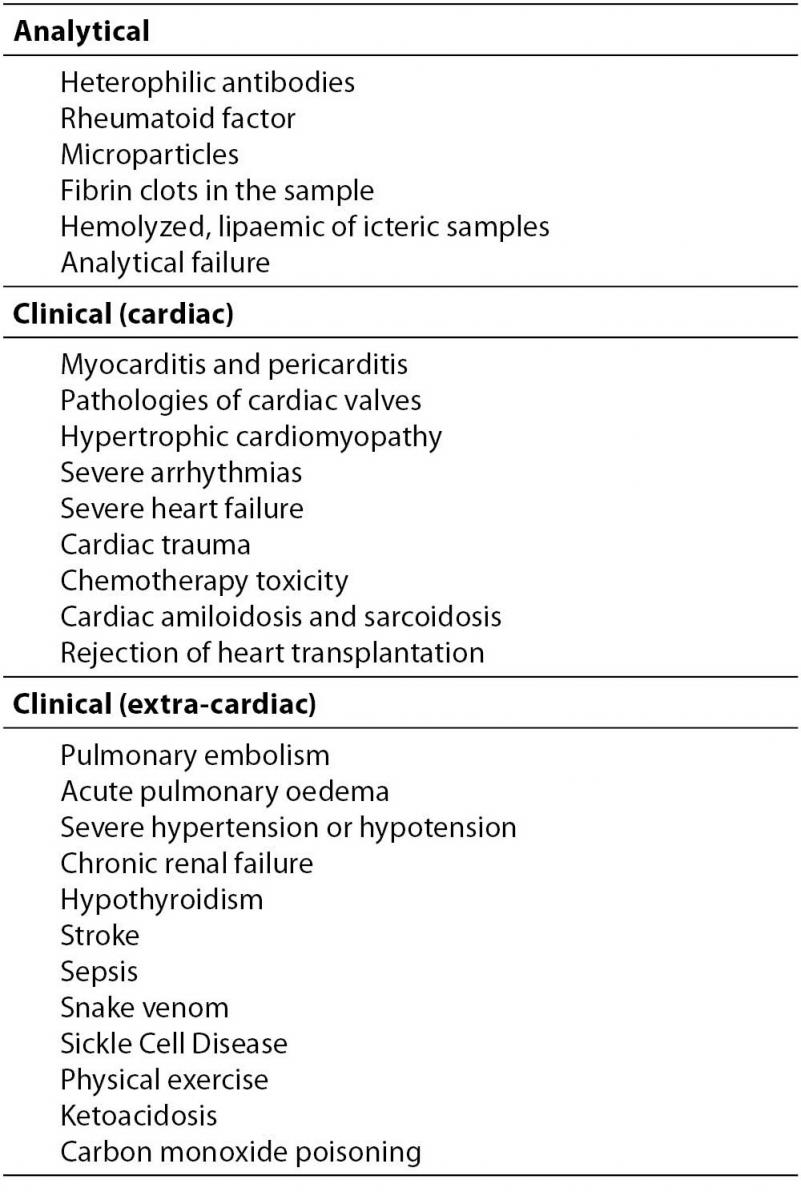
5. Dodig S. Interferences in quantitative immunochemical methods. Biochem Med 2009;19:50-62.
6. Lippi G, Plebani M. High-sensitive Troponin Testing and the „runner‘s Syndrome“. J Emerg Med. 2009 Aug 12. [Epub ahead of print].
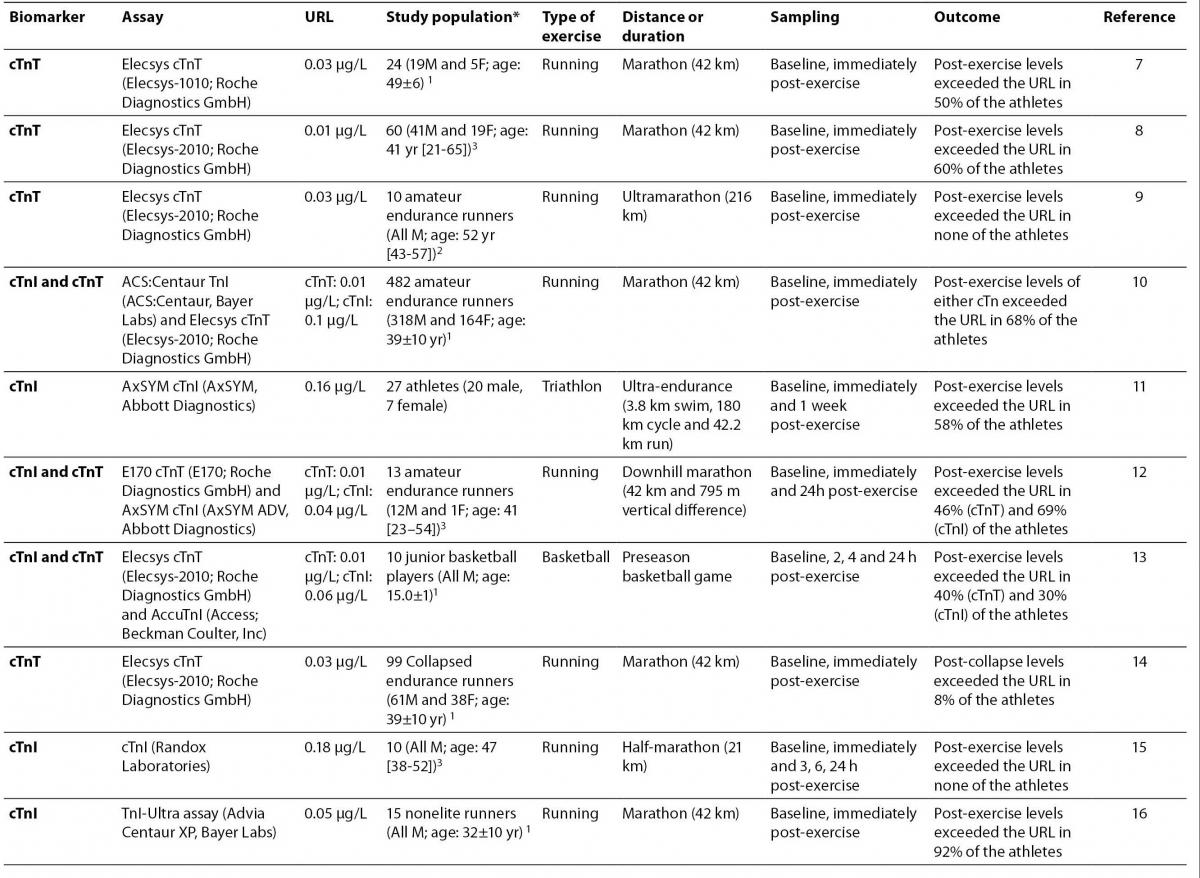
7. Melanson SE, Green SM, Wood MJ, Neilan TG, Lewandrowski EL. Elevation of myeloperoxidase in conjunction with cardiac-specific markers after marathon running. Am J Clin Pathol 2006;126:888-93.
8. Neilan TG, Januzzi JL, Lee-Lewandrowski E, Ton-Nu TT, Yoerger DM, Jassal DS, et al. Myocardial injury and ventricular dysfunction related to training levels among nonelite participants in the Boston marathon. Circulation 2006;114:2325-33.
9.Roth HJ, Leithäuser RM, Doppelmayr H, Doppelmayr M, Finkernagel H, von Duvillard SP, et al. Cardiospecificity of the 3rd generation cardiac troponin T assay during and after a 216 km ultra-endurance marathon run in Death Valley. Clin Res Cardiol 2007;96:359-64.
10. Fortescue EB, Shin AY, Greenes DS, Mannix RC, Agarwal S, Feldman BJ, et al. Cardiac troponin increases among runners in the Boston Marathon. Ann Emerg Med 2007;49:137-43.
11. La Gerche A, Connelly KA, Mooney DJ, MacIsaac AI, Prior DL. Biochemical and functional abnormalities of left and right ventricular function after ultra-endurance exercise. Heart 2008;94:860-6.
12. Koller A, Sumann G, Griesmacher A, Falkensammer G, Klingler A, Fliri G, et al. Cardiac troponins after a downhill marathon. Int J Cardiol 2008;129:449-52.
13.Nie J, Tong TK, Shi Q, Lin H, Zhao J, Tian Y. Serum cardiac troponin response in adolescents playing basketball. Int J Sports Med 2008;29:449-52.
14.Siegel AJ, Januzzi J, Sluss P, Lee-Lewandrowski E, Wood M, Shirey T, Lewandrowski KB. Cardiac biomarkers, electrolytes, and other analytes in collapsed marathon runners: implications for the evaluation of runners following competition. Am J Clin Pathol 2008;129:948-51.
15.Lippi G, Schena F, Montagnana M, Salvagno GL, Guidi GC. Influence of acute physical exercise on emerging muscular biomarkers. Clin Chem Lab Med 2008;46:1313-8.
16.Dawson EA, Whyte GP, Black MA, Jones H, Hopkins N, Oxborough D, et al. Changes in vascular and cardiac function after prolonged strenuous exercise in humans. J Appl Physiol 2008;105:1562-8.
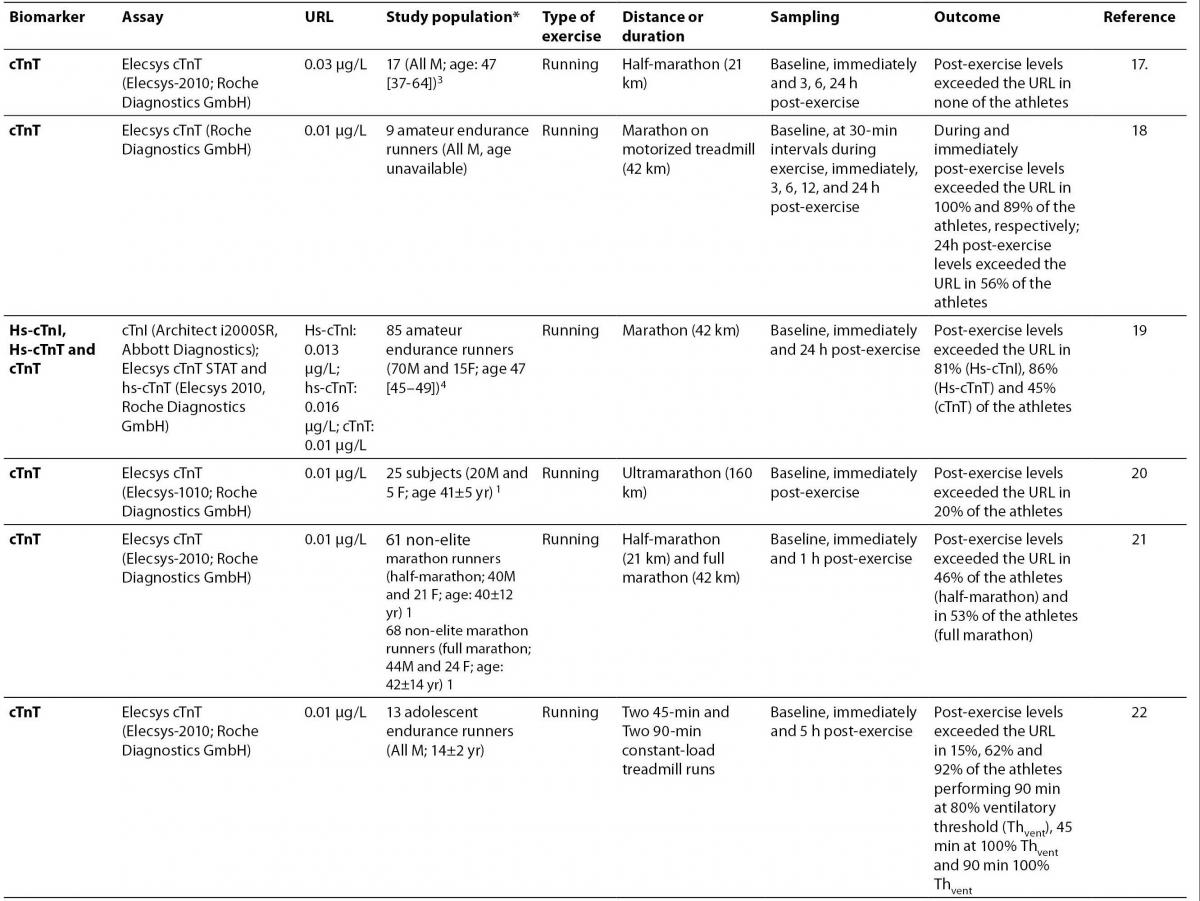
17.Lippi G, Schena F, Salvagno GL, Montagnana M, Gelati M, Tarperi C, et al. Influence of a half-marathon run on NT-proBNP and troponin T. Clin Lab 2008;54:251-4.
18.Middleton N, George K, Whyte G, Gaze D, Collinson P, Shave R. Cardiac troponin T release is stimulated by endurance exercise in healthy humans. J Am Coll Cardiol 2008;52:1813-4.
19.Mingels A, Jacobs L, Michielsen E, Swaanenburg J, Wodzig W, van Dieijen-Visser M. Reference population and marathon runner sera assessed by highly sensitive cardiac troponin T and commercial cardiac troponin T and I assays. Clin Chem 2009;55:101-8.
20. Scott JM, Esch BT, Shave R, Warburton DE, Gaze D, George K. Cardiovascular consequences of completing a 160-km ultramarathon.Med Sci Sports Exerc 2009;41:26-34.
21. Jassal DS, Moffat D, Krahn J, Ahmadie R, Fang T, Eschun G, Sharma S. Cardiac injury markers in non-elite marathon runners. Int J Sports Med 2009;30:75-9.
22.Fu F, Nie J, Tong TK. Serum cardiac troponin T in adolescent runners: effects of exercise intensity and duration. Int J Sports Med 2009;30:168-72.
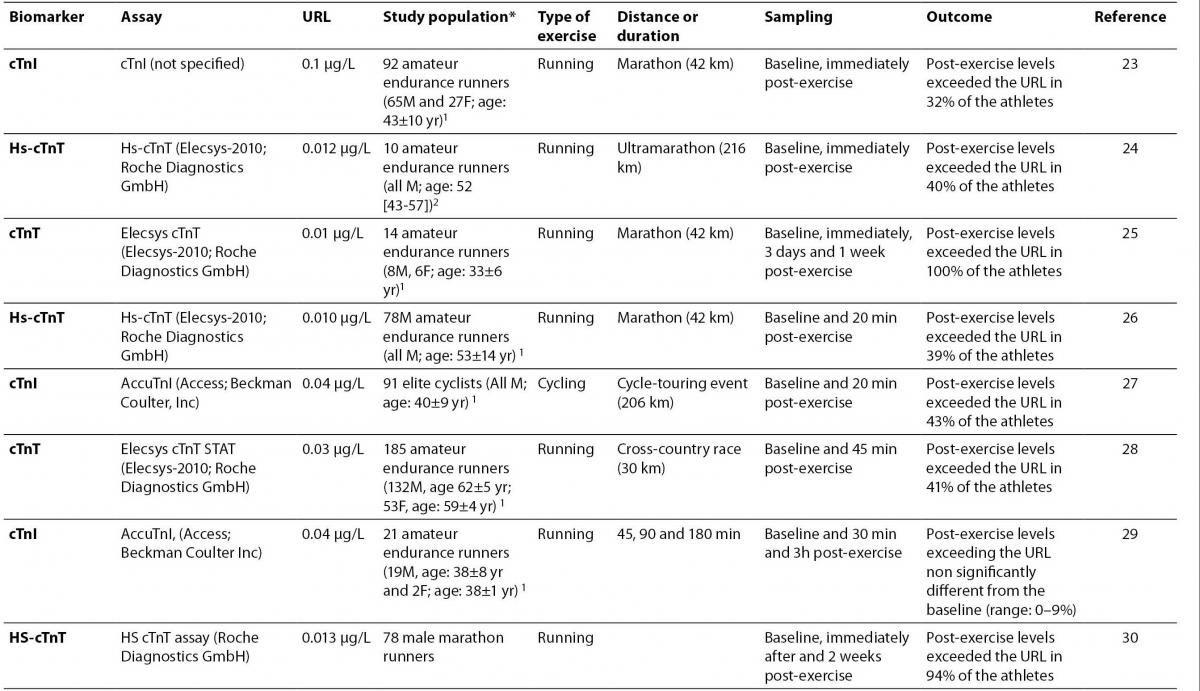
23.Hubble KM, Fatovich DM, Grasko JM, Vasikaran SD. Cardiac troponin increases among marathon runners in the Perth Marathon: the Troponin in Marathons (TRIM) study. Med J Aust 2009;190:91-3.
24. Giannitsis E, Roth HJ, Leithäuser RM, Scherhag J, Beneke R, Katus HA. New highly sensitivity assay used to measure cardiac troponin T concentration changes during a continuous 216-km marathon. Clin Chem 2009;55:590-2.
25. Mousavi N, Czarnecki A, Kumar K, Fallah-Rad N, Lytwyn M, Han SY, et al. Relation of biomarkers and cardiac magnetic resonance imaging after marathon running. Am J Cardiol 2009;103:1467-72.
26. Knebel F, Schimke I, Schroeckh S, Peters H, Eddicks S, Schattke S, et al. Myocardial function in older male amateur marathon runners: assessment by tissue Doppler echocardiography, speckle tracking, and cardiac biomarkers. J Am Soc Echocardiogr 2009;22:803-9.
27.Serrano-Ostáriz E, Legaz-Arrese A, Terreros-Blanco JL, López-Ramón M, Cremades-Arroyos D, Carranza-García LE, et al. Cardiac biomarkers and exercise duration and intensity during a cycle-touring event. Clin J Sport Med 2009;19:293-9.
28. Sahlén A, Gustafsson TP, Svensson JE, Marklund T, Winter R, Linde C, Braunschweig F. Predisposing factors and consequences of elevated biomarker levels in long-distance runners aged >or=55 years. Am J Cardiol 2009;104:1434-40.
29.Serrano-Ostáriz E, Terreros-Blanco JL, Legaz-Arrese A, George K, Shave R, Bocos-Terraz P, et al. The impact of exercise duration and intensity on the release of cardiac biomarkers. Scand J Med Sci Sports. 2009 Nov 17. ŠEpub ahead of printĆ
30. Saravia SG, Knebel F, Schroeckh S, Ziebig R, Lun A, Weimann A, et al. Cardiac troponin T release and inflammation demonstrated in marathon runners. Clin Lab 2010;56:51-8.
31. Regwan S, Hulten EA, Martinho S, Slim J, Villines TC, Mitchell J, Slim AM. Marathon Running as a Cause of Troponin Elevation: A Systematic Review and Meta-Analysis. J Interv Cardiol. 2010 Jul 20. ŠEpub ahead of printĆ.
32.Lippi G, Cervellin G, Plebani M. Sensitive cardiac troponin T assay. N Engl J Med 2010;362:1242.
33. Giannoni A, Giovannini S, Clerico A. Measurement of circulating concentrations of cardiac troponin I and T in healthy subjects: a tool for monitoring myocardial tissue renewal? Clin Chem Lab Med 2009;47:1167-77.
34. Hickman PE, Potter JM, Aroney C, Koerbin G, Southcott E, Wu AH, Roberts MS. Cardiac troponin may be released by ischemia alone, without necrosis. Clin Chim Acta 2010;411:318-23.
35. Trivax JE, Franklin BA, Goldstein JA, Chinnaiyan KM, Gallagher MJ, deJong AT, et al. Acute cardiac effects of marathon running. J Appl Physiol 2010;108:1148-53.
36. Wilson M, O‘Hanlon R, Prasad S, Oxborough D, Godfrey R, Alpendurada F, et al. Biological markers of cardiac damage are not related to measures of cardiac systolic and diastolic function using cardiovascular magnetic resonance and echocardiography after an acute bout of prolonged endurance exercise. Br J Sports Med. 2010 Jun 11. ŠEpub ahead of printĆ.
37. Lippi G, Banfi G. Exercise-related increase of cardiac troponin release in sports: An apparent paradox finally elucidated? Clin Chim Acta 2010;411:610-1.
38. O‘Hanlon R, Wilson M, Wage R, Smith G, Alpendurada FD, Wong J, et al. Troponin release following endurance exercise: is inflammation the cause? a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson 2010;12:38.
39. Lippi G, Guidi GC, Nevill A, Boreham C. The growing trend of scientific interest in sports science research. J Sports Sci 2008;26:1-2.