Introduction
Oxidative stress as a state of imbalance between oxidant and antioxidant processes of the human body may be both the cause and the consequence of many diseases. Due to this fact, the determination of oxidative stress, as well as the capacity of the antioxidant defence may be important for improving the health of individuals and of the general population.
Nowadays, it is a well-known that oxidative stress plays an important role in the pathogenesis of various kidney diseases. The kidney is an organ highly vulnerable to damage caused by reactive oxygen species (ROS), most likely due to the abundance of long-chain polyunsaturated fatty acids in the composition of renal lipids (1). Urinary tract infection (UTI), the most common bacterial infection that affects the human population, causes oxidative stress and leads to suppression of the antioxidant enzymes (2). Oxidative renal injury during UTI results partly from ROS generated by activated inflammatory cells, such as neutrophils and monocytes, which infiltrate the glomeruli (3). Polymorphonuclear leukocytes, a major limiting factor for bacterial growth in the urinary tract, are responsible for the development of renal tissue damage as well (4). In addition, it is a well-known that bacteria break down collagen and other components of the extracellular matrix, amplifying glomerular and mesangial damage (5). Moreover, associated with the degree of inflammation, release of cytokines and proteolytic enzymes from leukocytic infiltrates may initiate scar formation and renal fibrosis, subsequently resulting in an end-stage kidney disease (6,7). More severe forms of the UTI may also cause acute complications. Children with an UTI may develop a pre-renal type of acute kidney injury(AKI) because of renal hypoperfusion due to severe dehydration.
Scintigraphy is a current diagnostic procedure for monitoring of renal involvement during UTI (upper UTI) and related parenchymal inflammation. This is an invasive and expensive technique that is not always available, and a search for non-invasive methods therefore deems necessary (8). Motivated by the growing awareness of the impact that oxidative stress plays in the pathophysiology of kidney diseases, this study was created primarily with a desire to consider the role of new parameters and non-invasive method in assessing the condition and the outcome of a disease in paediatric patients with a diagnosis of UTI.
Due to extreme instability of ROS, the clinical assessment of oxidative stress is based on measuring of different stable oxidized by-products and antioxidant substances. Serum (or plasma) concentrations of different oxidants and antioxidants can be measured in laboratories separately, but the measurements are time-consuming, labor-intensive and require complicated techniques (9,10). Accordingly, many methods which measure total prooxidant and antioxidant capacity separately have been developed. Parameters determining the total content of oxidants and antioxidants may be especially useful in paediatrics, which often dispose of small amounts of specimens.
We tested the hypothesis that kidney damage during an UTI is associated with inflammation and increased production of oxidative free radicals in blood. The aim of this study was to determine the total prooxidant and antioxidant capacity of children with UTI by measuring parameters: total oxidant status (TOS), total antioxidant status (TAS), oxidative stress index (OSI), as well as their changes according to acute inflammation persistence and AKI development.
Materials and methods
Study design and subjects
We performed a prospective observational single-centre cohort study of children with UT at the University Children’s Hospital in Belgrade, Serbia, over a period of one year (between January 2011 and December 2011). The patients enrolled in the study consisted of 50 Caucasian children from 1 month to 12 years of age (median age was 6 months) with a first-time community-acquired UTI, of whom 28 were male patients and 22 were female patients.Three patients were between 3 and 12 years old, four of them were between 12 and 36 months and most of them, 43 or 86% were younger than 12 months. An informed consent was obtained from parents prior to enrolling children into the study. The consent was prepared according to the Declaration of Helsinki ethical guidelines. An institutional review committee approved our study protocol by following local biomedical research regulations. Children with known urogenital or anorectal malformations, or neurological disease were excluded from the study. After obtaining the informed consents at the time of admission to the hospital, urine culture, urinalysis, serum urea, creatinine, C-reactive protein (CRP) and white blood cell (WBC) count measurements were performed. Patients who met the following criteria were included in the study: fever higher than 38.5 °C, leukocytosis defined as leukocyte count more than the normal value according to age, CRP higher than 20 mg/L, positive reaction for leukocyte esterase in urine test strip and/or pyuria (microscopic examination of urine sediment: ≥ 10 WBC). UTI was diagnosed when there was significant bacteriuria (> 100.000 colony-forming units (CFU/mL)) in the urine culture. All children underwent abdominal and urinary tract ultrasound examinations within the 48 hours of admission to the hospital. However having a voiding cystourethrogram was optional (pending physician’s request and parents’ decision). Repeat laboratory tests (urine, urine culture, blood WBC and CRP) were performed on all patients after 48-72 hours of admission to the hospital. Discharge criteria were evaluated by analysing the clinical response to antibiotic therapy (fever, blood WBC and CRP, urine WBC and urine culture).
Classification of patients was carried out in two ways: assessment of the renal function, and of the duration of inflammation during UTI according to the CRP value upon discharge from the hospital. Assessment of the renal function was performed via estimated glomerular filtration rate (GFR) using the Schwartz formula (11):
GFR (mL/min/1.73 m2) = k x body height (cm)/creatinine (μmol/L);
coefficient k is 39.8 for full term infants ≤ 1 year, 48.6 for children aged 2-12 years and for female older than 12 years, and 61.9 for males older than 13 years. Grouping of patients according to AKI development (with AKI and without AKI, respectively) was performed according to the decline of GFR to below 25% in relation with the normal values for the age (12). In another classification, patients were divided by CRP value at discharge from the hospital. A concentration of CRP ≥ 10 mg/L is considered indicative of a clinically relevant inflammatory condition (13). In this study, it represents UTI in remission but with prolonged inflammation. Accordingly, two groups were formed:patients with CRP concentration higher than 10 mg/L were marked as patients with a longer duration of inflammation (coded with 1), while patients with CRP concentration less than 10 mg/L were marked as patients with a shorter duration of inflammation (coded with 0).
Sampling
Blood samples were drawn from each patient to measure the following parameters: WBC count, serum concentrations of inflammation marker CRP, renal function parameters - urea and creatinine, and oxidative status parameters – TAS and TOS. Blood was collected in vacuum tubes without additive (4 mL) for biochemical analysis and withanticoagulant K2-EDTA (3 mL; both Becton, Dickinson and Company, Franklin Lakes, NJ, USA) for WBC count. Centrifugation of clotted blood was performed in order to collect serum at 1200 x g for 10 minutes. Serum samples for oxidative status parameters were frozen at -80 °C and analysed within 3 months, while all other tests were performed immediately. Urine samples were collected by midstream clean catch or sterile bag method. One part of the urine was used for chemical analysis and microscopic examination of the urine sediment, and the other part of urine was transferred to dipslide, in order to ensure adequate storage for microbiological examination.
Methods
CRP was determined by a nephelometric method, Siemens C-reactive protein assay using a Dimension RxL Max Integrated Chemistry System (Erlangen, Germany). Creatinine and urea were analysed by routine methods, Olympus System Reagents using an Olympus analyzer AU 2700 (Hamburg, Germany). TAS was determined using a spectrophotometric method developed by Erel (9). This method is based on the decoloration of 2.2-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid)-radical cation by antioxidants present in serum. The reaction rate was calibrated with Trolox (a water-soluble analogue of vitamin E, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) and the TAS value of the samples is expressed as mmol Trolox equivalent per litre (mmol Trolox Equiv./L). TOS was determined using a colorimetric method also developed by Erel (10). The assay is based on the oxidation of ferrous ion to ferric ion in the presence of various oxidant species in acidic medium and the measurement of the ferric ion by xylenol orange. The assay is calibrated with hydrogen peroxide and the results are expressed in terms of micromolar hydrogen peroxide equivalent per litre (μmol H2O2 Equiv./L). Serum samples were measured spectrophotometrically at 620 nm for TAS and at 546 nm for TOS on ILab 300 Plus analyzer, Instrumentation Laboratory (Milan, Italy). The intra-assay and inter-assay coefficients of variation for TAS were (1.9% and 4.4%, respectively) and forTOS were (3.0% and 5.6%, respectively). The TOS to TAS ratio was regarded as OSI (14). To perform the calculation, the result unit of TAS, mmol Trolox equiv./L, was changed to μmol Trolox equiv./L, and the OSI value was calculated as follows:
OSI (arbitrary unit (AU)) = TOS, μmol H2O2 Equiv./L / (TAS, μmol Trolox Equiv./L / 100).
WBC count was performed at hematology analyzer Nihon Kohden MEK-7300K (Tokyo, Japan).In order to ensure adequate storage of urine for microbiological examination health care personnel used Dip-Slide,Liofilchem (Roseto degli Abruzzi, Italy).
Statistical analysis
Statistical analysis was done with MedCalc for Windows Version 9.6.3. (Mariakerke, Belgium) statistical software. The distributions of variables were checked for normality by Shapiro-Wilk test.Differences between subjects at the time of admission to the hospital and at the time of hospital discharge (two measurement points) were analysed by paired Student-t-test for normally distributed variables and by Wilcoxon signed-rank test for skewed distribution. Mann-Whitney U test was used to assess the changes of examined parameters according to duration of inflammation and AKI presence. Data are shown as mean ± standard deviation for normally distributed continuous variables and as relative of absolute frequencies for categorical variables. Variables with skewed distribution were expressed as median value and inter-quartile range. All tests were considered significant at P < 0.05.
Results
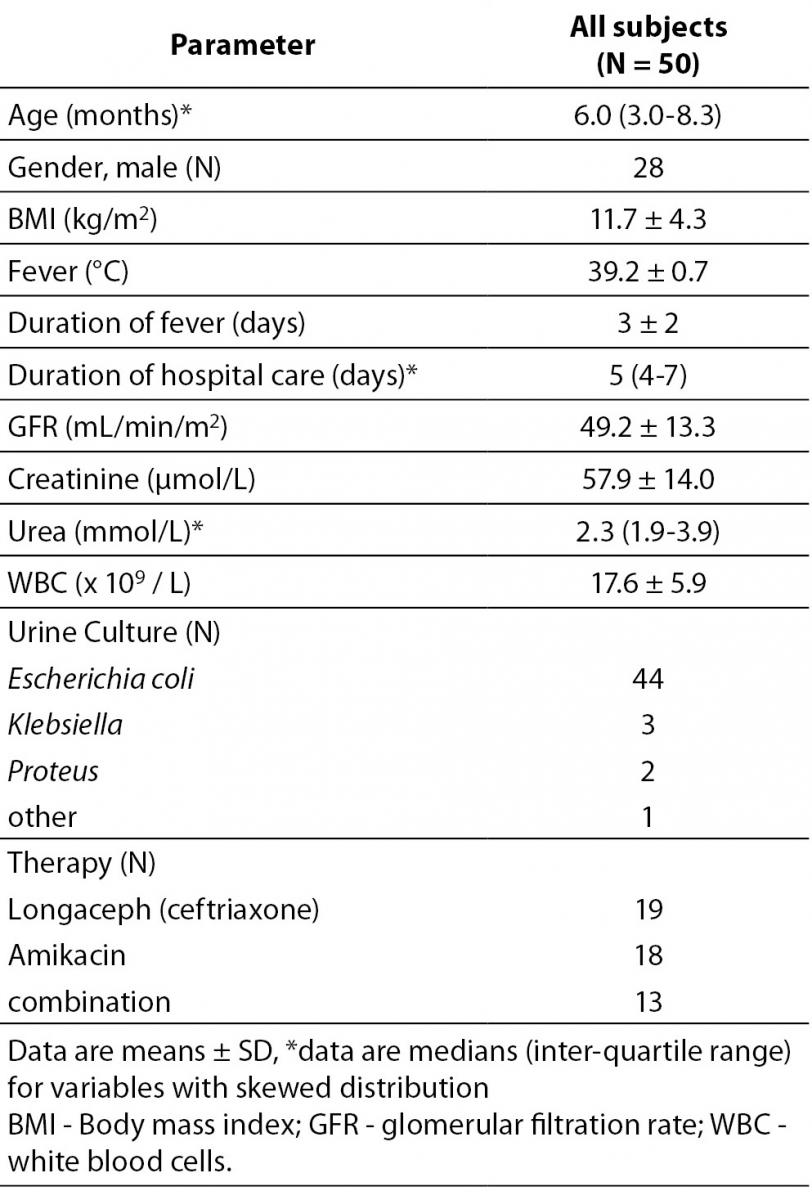
Baseline characteristics of the study population are presented in Table 1. As it might have been presumed, the cause of UTI in most cases was Escherichia coli (88%, 44 cases). The average length of the hospital stay was 5 days.
Table 1. Demographic and biochemical characteristics in the study population.
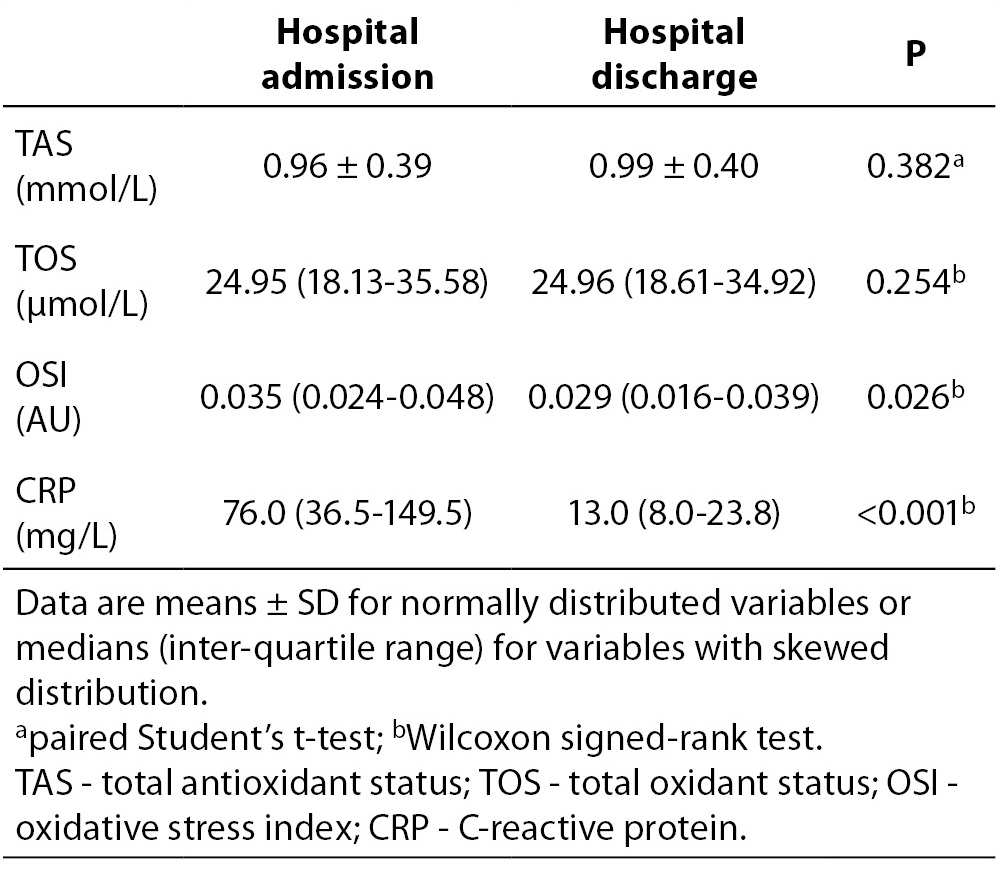
Oxidative status and inflammatory parameters of subjects at the time of admission to the hospital and at the time of hospital discharge are listed in Table 2. From oxidative status parameters only the OSI values were significantly lower at the time of hospital discharge than the corresponding values at the time of admission to the hospital (P = 0.026). In addition, the CRP concentrations were significantly lower at the time of hospital discharge than the corresponding values at the time of admission to the hospital (P < 0.001).
Table 2. Oxidative status and inflammation parameters of 50 subjects at the time of admission to the hospital and at the time of hospital discharge.
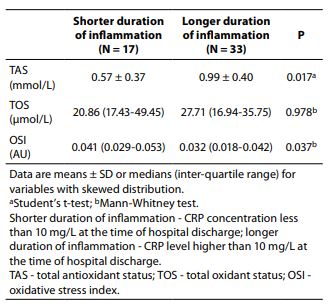
We used Mann-Whitney analysis in order to determine whether basal values of oxidative status parameters were changed according to duration of inflammation (expressed in relation to the concentration of CRP). TAS values were significantly higher in the subjects with longer duration of inflammation than in the subjects with shorter duration of inflammation (0.99 vs. 0.58 mmol/L, P = 0.017). Consequently, OSI values were significantly lower in the group with longer duration of inflammation than in the group with shorter duration of inflammation (0.032 vs. 0.041 AU, P = 0.037), Table 3.
Table 3. Differences in baseline values of oxidative status parameter according to duration of inflammation (expressed in relation to the concentration of CRP).
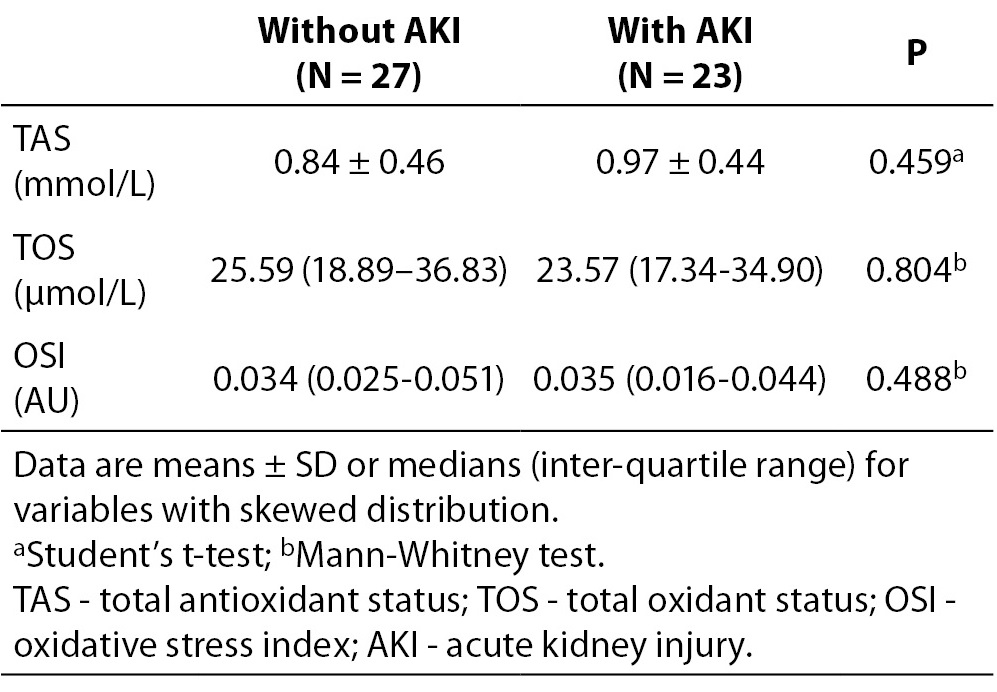
Furthermore, we analysed differences in basal values of oxidative status parameters according to AKI development. Obtained results are presented in Table 4. We did not find significant difference in basal values of oxidative status parameters between groups with and without AKI.
Table 4. Baseline values of TAS, TOS and OSI in children divided in two groups according to AKI development.
Discussion
This study analyses the behaviour of oxidative status parameters in children taking into account the duration of the inflammation and the risk for AKI development during UTI. To our knowledge, there are no published reports on serum oxidative stress parameters such as TAS, TOS and OSI in children with UTI. We have shown that the parameters TAS and OSI changed with the duration of inflammation, while only OSI reflected changing in the oxidative-antioxidant balance at discharge from the hospital. However, none of examined parameters were changed according to acute changes in kidney function.
As we can see from the two measurement points (at the time of admission to the hospital and at the time of hospital discharge), the TAS values were increased, while the TOS values were decreased in children upon discharge from the hospital, but this difference was not significant. However, when determining OSI, changes in both parameters TAS and TOS become evident. This is one more proof that in some pathological conditions useof an index that combines factors with different effects (for example apoB/apoA index, LDL/HDL index, etc.) may provide more advantages when assessing a disease.
Since the inflammation accompanies infections, at first we wanted to examine the parameters of oxidative stress, depending on the duration of inflammation. Condition of prolonged inflammation during UTI can lead to development of fibrosis and renal scar formation (8). Baseline values of TAS were higher in patients with longer duration of inflammation, which was confirmed by measuring the inflammatory markerCRP at the time of hospital discharge. A possible reason for this may be that although the serum contains different antioxidant molecules, proteins constitute the main antioxidant component of the serum. Free sulfhydryl groups of proteins are responsible mainly for their antioxidative effects. It was calculated that total protein contributes 52.9% to the measured serum TAS in healthy subjects (9). On the other hand, it is a well-known that the inflammatory response occurs with alterations in the expression of acute phase proteins, cytokines. Accordingly, higher values of TAS in conditions of prolonged inflammation are not surprising.
Furthermore, we observed if there was a change in the oxidative status parameters regarding the kidney injury. However, we have not proven that the oxidative status parameters were changed significantly in children with AKI as compared to children without AKI. A previous study in adult patients with chronic kidney disease, that measured certain parameters of plasma (TAS, creatinine, urea, uric acid) before and after dialysis, showed that decreasing TAS after dialysis mainly corresponded to diminution of low-molecular weight substances creatinine, urea and uric acid (15). A significant decrease in TAS values after dialysis was also determined in paediatric patients during hemodialysis (16). In our study, TAS did not reflect changes in concentration of antioxidant substances susceptible to renal function.
Also, it is necessary to consider that many pharmacological experimental studies have investigated the impact of radical scavengers and antioxidant vitamins (17-19), anti-inflammatory drugs (20), especially cyclooxygenase inhibitors (21), and combinations of these agents (17), in addition to conventional antibiotic treatment protocols, on renal scaring (22). Additional studies should be performed to examine the potential significance of TAS, TOS and OSI as parameters for the selection of patients who would becandidates for the application of additional therapy to antibiotics for the prevention of eventual complications during UTI. Previous study in children with primary nephrotic syndrome showed that TAS values could predict the response to corticosteroids at the time of diagnosis, while TAS values after weaning of corticosteroids could predict whether the patients would relapse or not (23).
In order to evaluate oxidative stress during UTI in paediatric patients, we measured parameters TOS and TAS easily and inexpensively with Erel’s method,and calculated OSI by applying mathematical operations division. We hope that after a detailed examination, parameters TOS, TAS and OSI would be included in the spectrum analysis of routine biochemical laboratory, and that their use will enhance the work of paediatricians.
The limitations of our study are a small study population and the absence of data controls. However, because of the design of the study, formation of a control group was not necessary. Unfortunately only indirect measures of renal parenchymal inflammation were assessed, as opposed to more direct assessment with upper urinary tract imaging studies such as scintigraphy which has been shown to be very accurate in the assessment of renal parenchymal inflammatory damage when compared directly to histopathology. Association between the oxidative status parameters and the more direct assessment of renal inflammation and subsequent scarring would have strengthened the findings of this study. Future studies should include direct imaging of acute inflammation, higher number of subjects, while the data of the control group will enable the definition of reference ranges for these parameters.
Conclusions
Our study demonstrated that OSI values could detect the simultaneous change of TAS and TOS due to change in the oxidative-antioxidant balance during the recovery of children with UTI. Parameters TAS and OSI changed with the duration of inflammation. In this way, our research confirmed that TAS and OSI could exhibit different trend values in relation to pathology to be tested, and pointed at the need for further investigations to ensure their adequate clinical applications and interpretation.We can conclude that TAS and OSI as a markers of oxidative stress during UTI are sensitive to accompanying inflammatory condition. However, none of examined parameters were sensitive to acute changes in kidney function. Further investigations are needed to evaluate whether TAS, TOS and OSI could be used to monitor disease severity in children with UTI.
Acknowledgement
This study was financially supported by grant from the Ministry of Education, Science and Technological Development Republic of Serbia (Project Numbers 175035 and 175079).