Insights into peer review
Since the very beginning of scientific publishing, peer review was important element for every editorial decision. In the last century, the leading medical journals like Science, Nature and Cell developed the system into the form that is nowadays known (1). Yet peer review is far from perfect. It has been argued that it is expensive, time-consuming, biased, inconsistent, conservative and often abused. Even though many researchers, editors and policymakers have questioned its objectivity and purpose, it is still most commonly used tool for objective judgment of submitted manuscripts (2,3). Not every journal submits their manuscripts for peer review, though many claim to do so. A growing number of the so-called “predatory” open-access journals falsely declare themselves as being peer-reviewed and committed to communicating high-quality research. Quite often, these journals require authors to pay a fee once the paper is accepted for publication. To expose the fraud behind this process, John Bohannon submitted a fabricated study with obvious errors in data analysis and interpretation to 304 open access journals under the false identity of Ocorrafoo Cobange. More than 60% of the journals accepted it, and nearly 60% of those acceptances came with no peer review evaluations (4). Of course, not every open-access journal fails to submit their papers for rigorous peer review. Nevertheless, this experiment exposed a serious flaw in peer review and raised numerous concerns about unethical and unprofessional editorial practice in great number of open-access journals.
These and other concerns have led some to suggest that “peer review system is in crisis” (5,6). Similar to the Bohannon experiment, Douglas Newton submitted similar manuscripts in several academic journals from the field of education. The heterogeneity in editor responses and reviewer comments led him to conclude that both reviewers and editors can be careless and biased (7).
These concerns have helped drive research into efficacy of peer review. Some studies have suggested that younger researchers from university hospitals tend to review manuscripts more rigorously and fairly, and that the best reviewers are researchers who have greater number of publications in high impact journals (2). However, relatively little is known about the overall value of peer review for ensuring the quality of published work (8,9).
Efforts for improvement of the existing peer review system have been made in form of various guidelines by leading experts in scientific journalism (10,11) and research ethics (12), as well as educational articles in journals (13). However, journals must ensure that these guidelines influence peer review practices, which implies the need for active education and guidance. To explore how often and in which way journals provide instructions for their peer reviewers, Hirst A et al. found that only 41 of 116 health research journals (35%) posted instructions for their reviewers on the journal website (14). Biochemia Medica supports those initiatives by introducing these guidelines for peer reviewers in order to improve journal’s overall quality.
Ethical responsibilities of reviewers – existing recommendations
Some journals rely on peer review primarily as a means of selecting high-impact manuscripts, while others take a more educational approach, using peer reviewer assessments to improve manuscripts. Either way, peer review is based on mutual confidence among editors, reviewers and authors. High-quality review requires honesty and as much transparency as possible between editors and authors. According to the Editorial Policy Committee of the Council of Science Editors, reviewers have several ethical responsibilities (10):
- All material under review is strictly confidential. The reviewer should never discuss manuscripts with anyone without prior approval of the editor. A reviewed manuscript can be used as material for mentoring young reviewers only with the editor’s permission. Every person included in the peer review process should be identified in order to receive appropriate recognition. In addition, the “Recommendations for the conduct, reporting, editing, and publication of scholarly work in medical journals“ of the International Committee of Medical Journal Editors (ICMJE) stated that all materials received during the peer review process should be destroyed after the review is submitted to the editor (11).
- If the reviewer feels that the manuscript goes beyond his expertise and that this may compromise the quality of his review, the reviewer should make that clear to the editor. Undertaking a review without proper competence can have a major effect on the outcome of the manuscript.
- If there is any interest that may impair objective review, the reviewer should excuse himself or disclose the potential conflict of interest to the editor, who will decide on the appropriate course of action. The reviewer may also be asked to sign a conflict of interest form, just as authors do.
- Reviewers should maintain their integrity. Their comments should be objective and impartial and should not be influenced by any personal data about the authors learned during the review process. Reviewers should not use any information they acquire during this process for their personal or professional benefit.
- When invited to review a manuscript, reviewers should always estimate whether the time given by the editor is likely to be sufficient. Reviewers should reply to the invitation as soon as possible, especially if they choose to decline. This gives the editor a chance to invite other reviewers and does not compromise deadlines during the review process.
- The reviewer’s role is not only to search for flaws and errors in the manuscript but also to point out its positive aspects and the value that it could provide to the journal. Every critique and comment should be based on objective evidence and include advice for improvement, written in a polite and constructive manner. Any suspicion of scientific misconduct should be reported to the editor and supported with strong arguments.
Models of peer review
By engaging into peer review, researchers take a large part in author’s success and professional advancement. Peer reviewers are meant to assess and encourage author adherence to high standard of research conduct and reporting, recognize and prevent scientific misconduct, and remain objective and impartial at all times. Many journals blind the identity of reviewers and/or authors in order to reduce unethical behaviour and biased reviews. However, the influence of blinding on the quality of the review process is debatable (9,15). There are several models of blinding, each with its own perceived advantages and disadvantages (Table 1) (3,16). Open review implies that both the reviewer and the author know each other’s identity. In a single-blind review, the reviewer knows author’s identity, but the reviewer’s identity is concealed. Double-blind review means that both the reviewer and the author are anonymous. Some journals are even expanding their conception of peer review to take into account the fact that the true review process starts after the publication of the article (7). There are some efforts in implementation of post-publication review or even the combination of pre- and post-publication review by encouraging discussion of articles through comments and ratings (PLoS journals, The Frontiers journals) or blogs (ResearchBlogging.org).
Table 1. Perceived advantages and disadvantages of peer review blinding models (3,15,16).
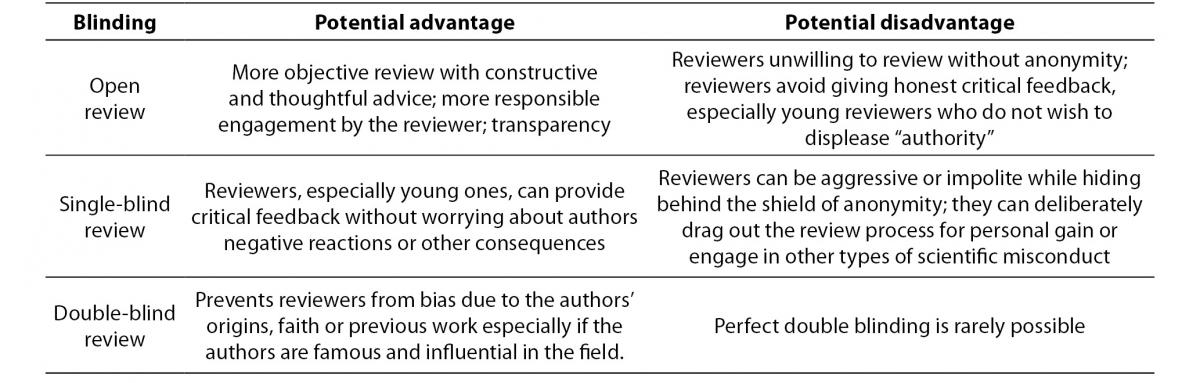
Biochemia Medica – peer review policy and guidelines
Biochemia Medica endorses the recommendations of various organizations playing a key role in promoting integrity of scholarly publications: the Committee on Publication Ethics (COPE), the International Committee of Medical Journal Editors (ICMJE), the Council of Science Editors (CSE), the European Association of Science Editors (EASE), and CrossRef. As a result, Biochemia Medica strives to implement these standards through education of authors, readers, editors and reviewers. Our goal is to conduct an honest and thorough editorial process, transparently declare our expectations from our reviewers, educate authors and provide high-quality scholarly material for our readers. We believe that this is our responsibility to our readers, authors and reviewers, as well as to the broader scientific community (17,18).
The editorial process in Biochemia Medica typically proceeds as follows. The Editor-in-Chief screens manuscripts to assess their correspondence with the journal’s scope and to evaluate their overall quality. This step is crucial for eliminating manuscripts unlikely to pass peer review, allowing journal reviewers to focus on manuscripts with greater chances of success. When found suitable by the Editor-in-Chief, manuscripts are then analyzed by the Research Integrity Editor, who reports back to the Editor-in-Chief on text similarity analysis (19). This step reduces the burden on peer reviewers to detect possible scientific misconduct, though reviewers are still obliged to report any misconduct if they suspect it. The Editor-in-Chief then assigns the manuscript to an Assistant Editor, who sends it out for peer review.
Biochemia Medica usually requires at least two peer reviews before making a decision about a submitted manuscript. More reviewers are sometimes needed, such as when the subject matter or statistical analysis requires additional expertise. Every reviewer is required to update the journal’s database with information about competencies and areas of expertise. Assistant Editors draw from this database to select reviewers likely to give high-quality review.
Biochemia Medica uses double-blind peer review. Therefore, as stated in the Instructions to Authors, the manuscript file should not contain the names or affiliations of the authors. All personal data should be reported on a separate document submitted as the title page.
Every invitation sent to the reviewer includes a brief explanation of his responsibilities. When responding to the invitation, each reviewer is asked to report any conflict of interest by emailing a short statement to the editorial office. The invitation also contains a link to a reviewer’s check list to help ensure accurate and comprehensive review (Table 2). Since Biochemia Medica aims to educate authors and improve manuscript quality, reviewers are encouraged to write detailed reviews with thorough explanations whenever possible.
Table 2. Checklist for reviewers of manuscripts submitted to Biochemia Medica. This checklist is intended only to provide guidance; reviewers are not obliged to answer all questions, especially if they feel they lack the necessary competence.
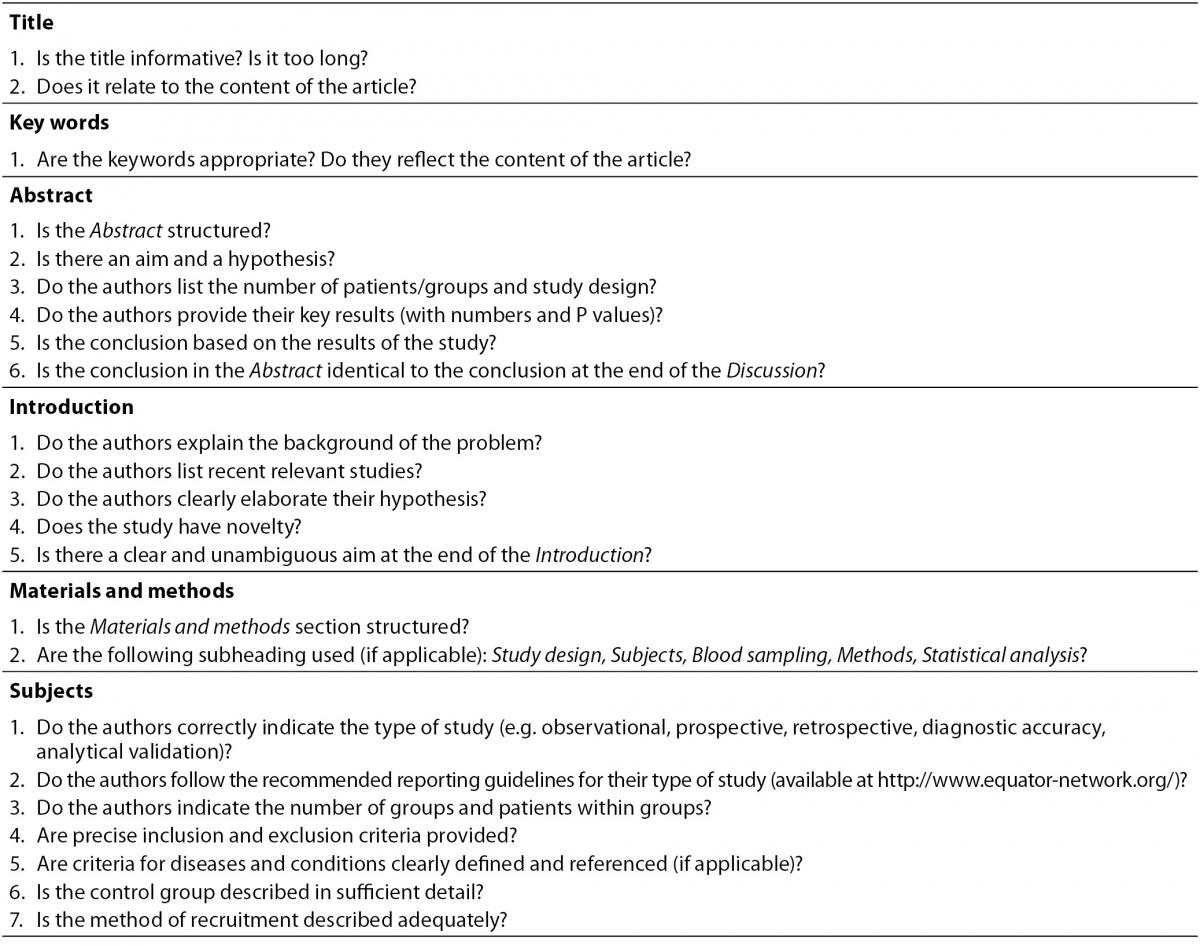
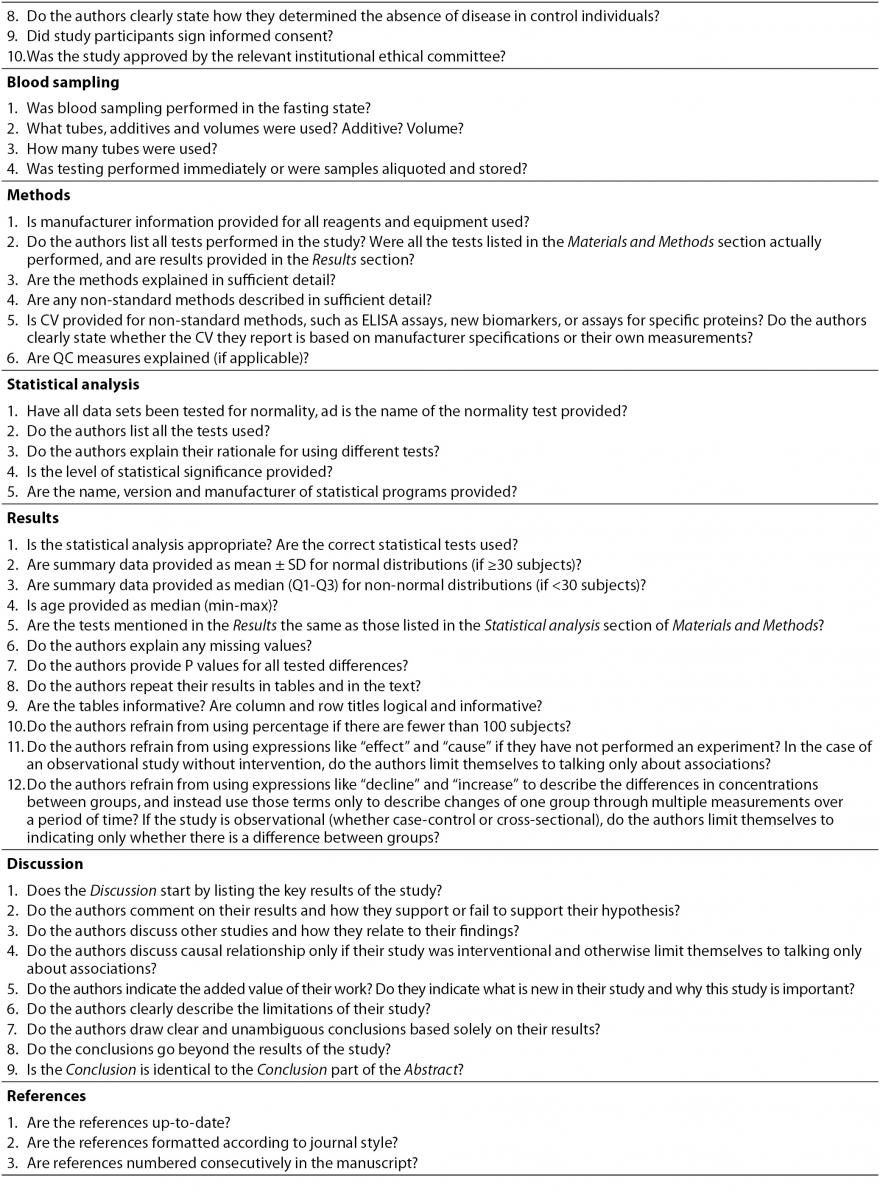
When receiving an invitation from Biochemia Medica, the reviewer is supposed to:
- Read the manuscript abstract included in the invitation in order to evaluate whether the reviewer has the necessary expertise in the subject area involved. The reviewer should accept or reject the invitation as soon as possible. If for any reason, during the review process, the reviewer feels he has insufficient expertise to handle the manuscript, the reviewer should inform the editor promptly.
- Respect the deadline for responding to the invitation as well as the deadline for submitting the review. Failing to respect these deadlines is considered a lack of professional courtesy. Reviewers should contact the editors promptly if they require an extension.
- Declare any possible conflict of interest to the editor. If potential conflicts of interest appear during the review process, they should be reported accordingly.
- Keep all materials provided by the journal strictly confidential. The editor must approve any sharing of the material with a third person. All persons involved in conducting the review should be properly identified.
When writing a review, the reviewer is supposed to:
- Read the Journal’s scope and Instructions to Authors in order to write the review with journal objectives and format guidelines in mind.
- Consult the journal’s Guidelines for Reviewers.
- Evaluate the manuscript objectively and impartially. Perfect blinding of the manuscript is not always possible; sometimes personal information about the author or the author’s institution can be surmised from the manuscript content.
- Notify the editor about any doubt in the integrity of the manuscript content or conduct of the study. The reviewer should never investigate potential misconduct by himself.
- State the overall recommendations for the manuscript: accept, accept after minor modifications, accept after major modifications, reject.
- Provide additional comments according to the Journal’s checklist (Table 2). The checklist is aimed to prevent the omission of important issues and to help reviewers structure their reports. The checklist is only for guidance; reviewers are not obliged to answer all the questions, especially if they do not feel competent to do so.
- Summarize all the positive findings of the manuscript that could provide value to the journal.
- Write every criticism or comment in clear, concise and polite language. State the exact sentence in question by citing page number, text line or paragraph. For example: “Page 4, line 14: instead of ’preanalytical warnings’ the author could consider using the phrase ‘preanalytical recommendations’.”
- Follow up all criticisms with explanation and advice for correction. If appropriate, comments should be supported with evidence from the literature. For example, instead of merely stating “The statistical test used in the text is wrong.” consider giving an explanation with advice for further reading: ”Correlation is not tested using the appropriate statistical test. The statistical test for correlation depends on sample size and normality of data distribution. For large samples and normally distributed data, the Pearson correlation test should be used. In the case of a small sample or deviation from normal data distribution, consider using the Spearman correlation test.”
- Never address the author personally, make any improper comments or use aggressive terms. Moreover, it is highly inappropriate to use capital letters, exclamation marks or direct verbal insults. For example, instead of “THIS STATEMENT MAKES NO SENSE!!!”, it is much better to write: “The authors may consider rephrasing the sentence.”
- Strive to make the review educational so the author can learn from his mistakes and improve the manuscript as much as possible. Instead of criticizing theDiscussion section of the manuscript, try to explain the proper way for discussing the results, such as: “To improve the Discussion I suggest that authors start with their key findings, discuss their results in comparison with existing data in the literature, and explain how their results fit into what’s already known. I also strongly suggest that authors emphasize the added value of their results and the novelty of the study.”
- Inform the editor about all limitations of the review in the section “Confidential comments to the editor”, such as if the reviewer does not feel confident enough to review the statistical analyses.
After submitting the review, the reviewer is supposed to:
- Destroy all copies of the reviewed manuscript in order to maintain confidentiality.
- Refrain from using any information acquired during the review process until after the article has been published.
- Read the reviews of other reviewers and contact the editor if additional comments are necessary.
Conclusion
Each published article is a combined effort of the authors, editors and reviewers, each of whom has his own responsibilities. The reviewer’s responsibility is to analyze the manuscript objectively and thoroughly and to provide useful advice and constructive comments to the author. Reviewers contribute significantly to the final editorial decision, but to do so properly, they should be honest and fair in their evaluations. Peer reviewers indeed carry great responsibility despite working for the journals as volunteers, but this noble activity does provide some benefits. Having the privilege of reading the “unknown” is thrilling, and helping to shape new information for presentation to the broader community provides a sense of importance. Reviewers also derive professional benefits: in many countries, including Croatia, peer review activities are taken into account for professional advancement in academic career. To ensure that the work of reviewers is recognized, Biochemia Medicapublishes, at the end of the year, the list of reviewers who contributed to the journal.
Reviewers play a fundamental role in maintaining the quality of research publications and how they are implemented in everyday lives. We thank all our reviewers for investing their time, effort and expertise in our journal. The editorial work, though demanding by itself, would be almost impossible without the valuable contribution of peer reviewers.


