Introduction
Multiple sclerosis (MS) is the most prevalent neurological disorder diagnosed in young adults, although no age group is exempt. Approximately 400,000 MS patients have been recorded in the USA and about 2.5 million worldwide, with an estimated incidence of 10,000 in the United States (1). MS is characterized by remission and exacerbations. Duration and symptoms of active disease vary greatly from patient to patient. Some enjoy a prolonged remission while others may fare poorly (1). The disability of such a productive population is the extra-motivation behind the efforts to understand and cure MS.
Pathologically, MS is characterized by death of oligodendrocytes, the cells engaged primarily in production of myelin, thereby myelin loss and presence of macrophages filled with degradation end-products. A striking feature at the lesion site during both the acute and chronic phase of the disease is iron accumulation. Although iron accumulation in the brains of MS patients has been known for a long time, the exact underlying mechanism is still obscure (2). Whether increased brain iron is the cause or only the consequence of a primary initiating factor is a dilemma. Independently of its source, free iron in a lipid rich, well oxygenated tissue means one thing: oxidative damage. The myelin sheath, which is 80% lipid in composition, is apparently vulnerable at the first place. Besides a plethora of possible causative factors, this uncommon pathologic feature supports the pivotal role of oxidative stress in MS. The rate of production of oxidative substances and their destruction is in a state of balance in a living organism. When this balance is shifted towards oxidants, the new condition is called oxidative stress. Although oxidative stress plays a role in the pathogenesis of every human disease to some extent, MS is distinctive that the source is evidenced by accumulated iron deposits detected by radiological and post-mortem pathologic studies (2).
Recent literature in MS seems to be dominated by papers dealing with rapid improvements in radiological imaging procedures. Advanced MRI techniques have made it possible to reliably measure iron content in brains of patients (3,4). Excess iron is a straight source of oxidative damage, and monitoring the iron status in MS patients might be of paramount importance. Multiple studies have already found an association between iron levels and various measures of disease disability (5).
Besides the rapid development in radiological imaging procedures, serum biochemistry markers do still bare every advantage of feasibility and cost effectiveness. The release of the commercially available new generation ready-to-use serum marker kits of oxidative stress caused an explosive increase in literature pertaining to the subject. “Oxidative stress hunters” raced to report “impaired oxidative balance” in every single human disease. Measurements of total antioxidant status (TAS) and total oxidant status (TOS) can provide information on an individual’s overall serum anti-oxidative status, which may include those oxidants and antioxidants not yet recognized or not easily measured (6,7). Albumin is a major determinant of the antioxidant capacity of human serum. The molecule owns the ability to bind and carry radical scavengers, and sequester transition metal ions with pro-oxidant activity besides direct anti-oxidant capabilities. The generation of reactive oxygen species (ROS) and free radicals can transiently modify the N-terminal region of albumin and produce an increase in the concentration of ischemia modified albumin (IMA) (8). Recent studies present IMA as a marker of ischemia and oxidative stress (8,9).
After all, measuring the status of oxidative balance in the body might be superior to measure barely the iron content, as it is not the iron load but the oxidative damage that generates the disease. Accordingly, the aim of this study was to measure TAS, TOS as general markers of the oxidative status and IMA levels as a marker of protein modification due to oxidative stress in RRMS patients.
Materials and methods
Subjects
Thirty-five stable relapse remitting MS (RRMS) patients (20 females and 15 males; median age 42 (20-55) years) admitted to the Antalya Education and Research Hospital were prospectively included into the study. All patients were clinically definite MS, according to the criteria of McDonald (10). Clinical disability and severity were scored using Kurtzke’s Expanded Disability Status Scale (EDSS) (11). The duration of the disease was expressed in years from the date of neurological diagnosis. The progression index (PI), defined as the ratio between EDSS/MS duration, was assessed in the entire MS group.
The control group consisted of thirty-five healthy volunteers matched for age, gender (22 females and 13 males; median age 37 (21-60) years), and geographic origin with the MS patients. Control subjects were without any sign or familial history for neurological diseases.
The patients were evaluated and diagnosed by the same neurologist on the basis of neurological examination. Patients with probable MS or clinical isolated syndrome were excluded from the study. In addition, those with any other acute or chronic inflammatory disease, anaemia, malignancy, cerebrovascular disease, pregnancy, or patients using any antioxidant drugs that could influence the results, were excluded. All participants belonged to the same ethnic group and had comparable socioeconomic status. All subjects had a full physical examination and were asked to complete a general questionnaire and gave informed consent before the onset of study. The following data were recorded for each patient: age, gender, medical history, alcohol consumption, and smoking. We asked for regularity in alcohol consumption. We defined anybody who smoked one or more cigarettes per day as a smoker.
The study was approved by the local Ethical Committee and all the recruited subjects signed an informed consent to participate to the study.
Samples
Blood samples were obtained after an overnight fasting. Five millilitres of blood from each patient and control was drawn into a BD Vacutainer (Becton-Dickinson, USA) SSTTM II Advance tube. Serum tubes are coated with micronised silica particles which activate clotting. Samples were checked for haemolysis or other interfering substances. Serum was then separated from the cells by centrifugation at 1500 x g for 10 minutes. Serum albumin was measured freshly. Remaining serum portions were stored at -80 °C and used to analyze TAS, TOS and IMA.
Methods
Reduced cobalt to albumin-binding capacity (IMA level) was measured using the rapid and colorimetric method developed by Bar-Or et al. (8). Briefly, 200 μL of patient serum was transferred into glass tubes and 50 μL of 0.1% CoCl2 x 6H2O (Sigma-Aldrich, Missouri, USA) added. After gentle shaking, the mixture was incubated for 10 minutes to ensure sufficient cobalt albumin binding. Then, 50 μL of 1.5 mg/mL dithiothreitol (DTT) (Sigma-Aldrich, Missouri, USA) was added as a colouring agent. After 2 minutes, 1 mL of 0.9% NaCl was added to halt the binding between the cobalt and albumin. A blank was prepared for every specimen. At the DTT addition step, 50 μL of distilled water was used instead of 50 μL of 1.5 mg/mL DTT to obtain a blank without DTT. The absorbances were recorded at 470 nm with a Shimadzu UV1601 spectrophotometer (Tokyo, Japan). Colour formation in specimens with DTT was compared with colour formation in the blank tubes, and the results were expressed as absorbance units (ABSU).
The inter-assay variability of IMA method in our laboratory was calculated from serum samples of 20 healthy subjects and 20 patients with acute coronary syndrome. The within-run coefficient of variation was 1.79% for healthy subjects (mean = 0.334; standard deviation = 0.006) and 1.62% for acute coronary syndrome (mean = 0.370; standard deviation = 0.006). IMA results should be interpreted with caution when serum albumin concentrations are < 20 g/L or > 55 g/L (12). The formula IMA value/individual serum albumin concentration was used to maintain the IMA rate (IMAR), in order to avoid the impact of albumin concentration differences between groups.
The TAS and TOS of the serum were measured by commercially available kits (Relassay®, Turkey) with an Architect ®c16000 auto analyzer (Illinois, USA), using an automated colorimetric measurement methods for both statuses developed by Erel (6,7). In TAS method, antioxidants in the sample reduce dark blue-green coloured 2,2’-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid) (ABTS) radical to colourless reduced ABTS form. The change of absorbance at 660 nm is related with total antioxidant level of the sample. Using this method, the anti-oxidative effect of the sample against the potent free radical reactions initiated by the produced hydroxyl radical, is measured. The results are expressed as micro molar trolox equivalent per litter.
In TOS method, oxidants present in the sample oxidize the ferrous ion–chelator complex to ferric ion. The ferric ion makes a coloured complex with chromogen in an acidic medium. The colour intensity, which can be measured spectrophotometrically, is related to the total amount of oxidant molecules present in the sample. The results are expressed in terms of micro molar hydrogen peroxide equivalent per litter (μmol H2O2 Equiv./L).
The variability in TAS and TOS measurements in our laboratory was calculated in 10 serum samples. The within-run coefficient of variation was lower than 3% for both parameters (TAS 2.6%, TOS 2.9%).
Statistical analysis
Statistical analyses were carried out using the statistical software version 11.5.1.0 (MedCalc®, Mariakerke, Belgium). Nonparametric variables were evaluated by using Chi-square and Fisher’s exact tests. Kolmogorov-Smirnov test was used to determine normality in parametric variables. All groups were found to be normally distributed, so the results were presented with mean and SD. The significance of the differences between these groups was determined by Student’s unpaired t-test. Pearson correlation coefficient was used to test the strength of any associations between different variables. P values equal or less than 0.050 were considered statistically significant.
Results
There were no significant differences in age, gender, smoking habits and body mass index (BMI) parameters between patients and controls. The patients were classified as RRMS and the disease duration ranged from 1 to 20 years with a mean ± SD of 7 ± 5 years. The scores of the EDSS ranged from 1 to 5 and the PI ranged from 0.04 to 1.2. The demographic and clinical data are summarized in Table 1.
Table 1. Demographic and clinical data of patients with MS and controls. The two groups were quite identical in age, gender, BMI and number of smokers
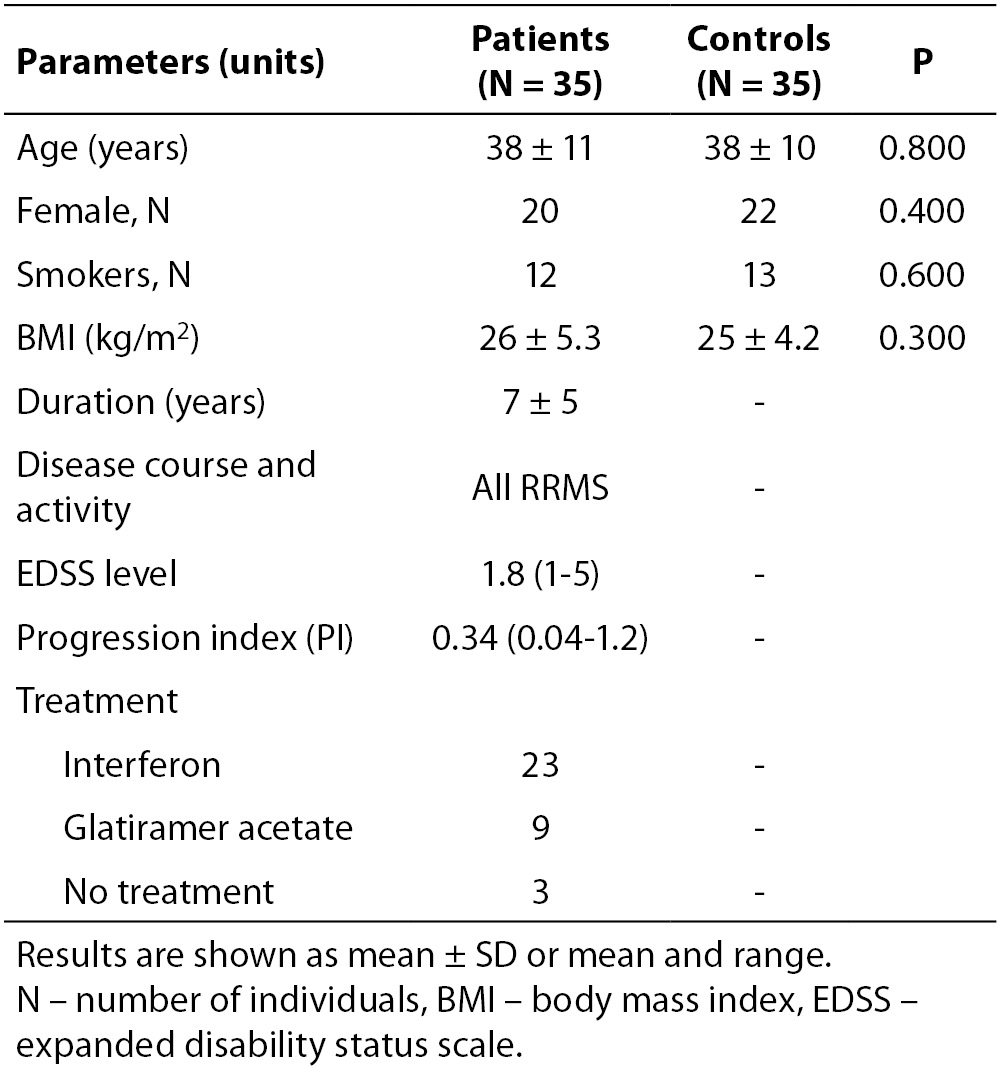
Table 2. Lower serum albumin in MS patients was representative of a chronic disease. IMA and IMAR measurements showed significant differences between RRMS group and controls
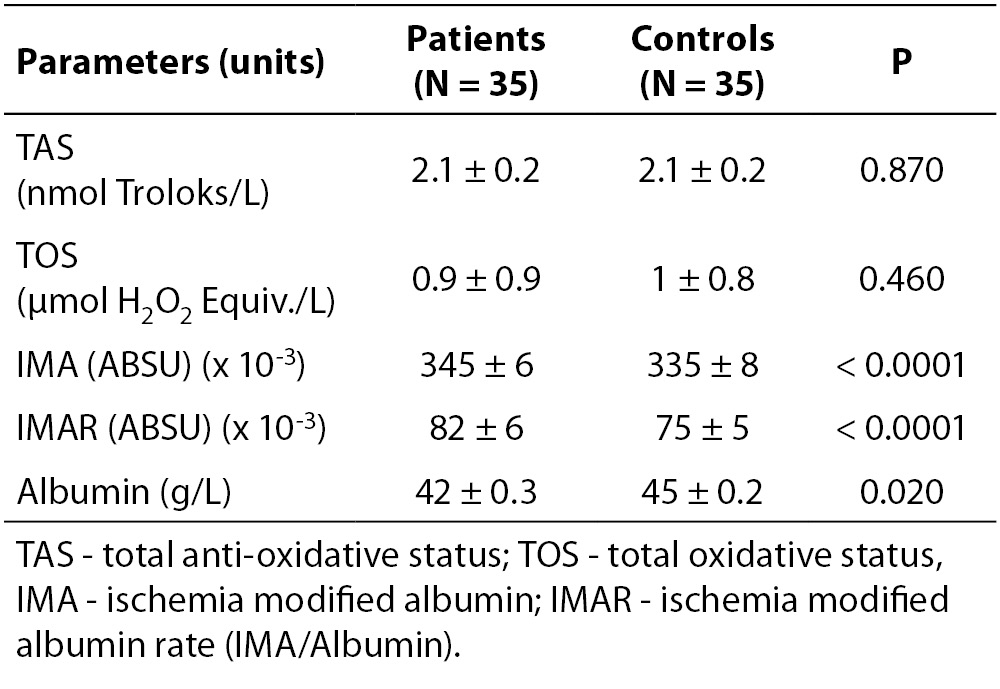
Serum albumin was lower in the patient group (P = 0.020), reflecting the state of a chronic disease (Table 2). IMA levels and IMAR were significantly higher in RRMS patients (P < 0.001 for both) (Figure 1), while TAS and TOS did not show any significant difference between groups (P = 0.870 and P = 0.460, respectively) (Table 2).
Correlation analysis was used to assess correlation between oxidative stress markers and disease duration, expanded disability status scale and progression index. There was no statistically significant correlation between these parameters.
Discussion
IMA levels and derived IMAR were significantly higher in RRMS patients, while TAS and TOS levels between RRMS patients and our control group did not show a significant difference. There are at least two explanations that fit to our results. The MS patients in remission were as healthy as our controls in means of oxidative stress, and IMA results were non-specific or false. Or, we were unable to measure the oxidative stress in these patients with TAS and TOS, per contra to IMA, which showed a remarkable difference between groups. In order to reach to the correct conclusion we tried to eliminate one of the two proposals.
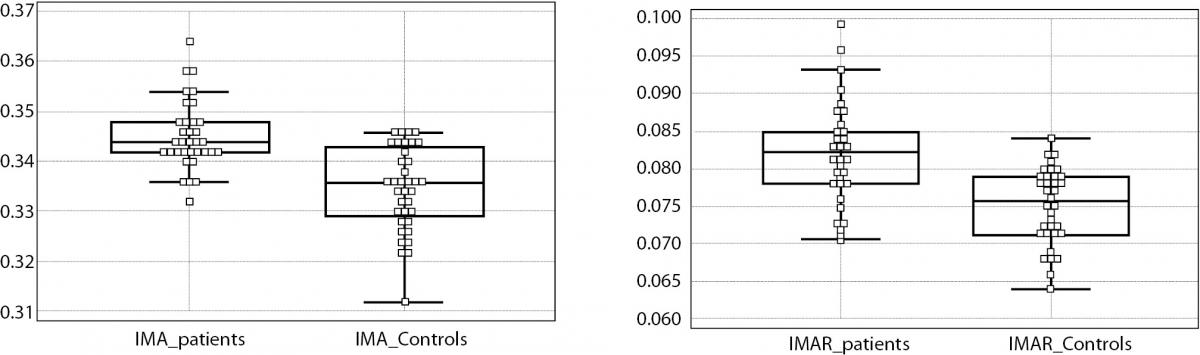
Figure 1. IMA levels were significantly higher in RRMS group than the controls (left). The difference was much more prominent after IMA values were divided by the individuals albumin value to maintain a rate, namely IMA rate (IMAR) (right).
MS patient in remission may be apparently healthy, but even in the state of remission the accumulated iron in the brain of these individuals is indolently, yet doggedly persistent. To appreciate the obvious consequences of this accumulation the basics of reactions between oxygen and transition metals should be reviewed (12). Quite a long time ago, when evolution has chosen a life with molecular oxygen, transition metals were automatically included in the process. In nature, where transition metals and oxygen interfere freely, ferrous iron will react with molecular oxygen to produce ferric iron. The reaction is energetically favourable and occurs spontaneously. Aerobic living organisms learned to benefit from the addiction of oxygen to metals by time. However, free interaction of metal and oxygen leads by-products that are destructive to organic structures. To avoid such untoward consequences, transition metals are kept in cage molecules like haem, chlorophyll or vitamin B12, where metal is fixed by coordination bonds between the metal and nitrogen. A free reaction is so terrifying that the metal in cage is also embedded in huge protein beds like haemoglobin. Despite all precautions however, production of unwanted products, namely radicals, is inevitable. To cope with this inevitable error, which is called oxidative stress, the human body is equipped with a number of endogenous scavenging systems. Unfortunately, these systems have limits, and these limits get lower with age and certain environmental factors like sedentary life style or smoking (13). The body works on a fragile balance between production of oxidants and their removal. Failure of the latter is the way to a state of disease of any kind, depending on the background of the individual.
A disease with extreme levels of iron deposition may serve to comprehend the catastrophe. Thalassemia patients are due to increment in serum and intracellular iron at extreme levels because of continuous blood transfusions. Due to aforementioned mechanisms, these patients are under a huge oxidative stress. To note, most effected organs are ones with a high content mitochondria, the unit of oxidative energy production, and thereby greatest sources of radical leak. Thus, it is not the iron accumulation but the oxidative stress it produces that damages the tissues. Unsurprising, Cakmak et al. found significantly lower TAS, TOS in patients with thalassemia major compared to a control group with P values lower than 0.0001 (14).
There is also a problem in perception of iron content as a parameter to monitor in managing MS patients. To render a correct point of view, we must differentiate “free iron” and “iron under control” as components of disease initiators. From the results of certain studies, we understand that MS disease process includes at some point a shift of cellular iron storage from oligodendrocytes to microglia and macrophages. Oligodendrocytes, highly dependent on iron availability for their normal functionality, are known to be the major iron-containing cells in the adult CNS. Of course, the iron in oligodendrocytes is in strict physiologic control. However, iron at the site of MS lesion is kept in microglia/macrophages. Indeed, iron enriched oligodendrocytes are a sign of healthy tissue, while iron-enriched microglia may indicate the presence of ongoing disease (2). This shift of iron from physiologic to pathologic deposition is the basic of our assumption that measuring oxidative stress is superior to mere measurements of iron load.
In a previous study, Kirbas et al. found significantly lower TAS and higher TOS in RRMS patients (15). We used the same kits as them; material and methods of both studies were quite the same. Therefore, we do not have an explanation to the contradiction. Measurement of TAS and TOS are valuable, as they provide information about the “totals” of oxidants and antioxidants including the ones not yet recognized or not easily measured in a medium. However, their “sum of all” nature is also their weakness, that they carry the risk of missing subtle changes. Thereby, these markers may fail in achieving the success they show in a disease with extreme changes like thalassemia, when considering a disease with subtle changes like the MS in remission.
Albumin shows structural modifications in respond to environmental changes in serum, including the increase of ROS. Such modifications are not unique to albumin, on the contrary are basics of some methods of oxidative stress measurements. Studies that have presented higher oxidative modification of plasma proteins (16,17), higher lipid oxidation products and oxide nitric metabolites (17,18), lower thiol levels (17,19) in MS patients compared to controls. One particular study that attracted our attention was by Fiorini et al. in which they preferred to use purified serum samples to measure oxidative stress markers and other proteins. Their standpoint for purification was to avoid high abundant proteins mask the subtle changes in the less abundant ones. Only after purification they were able to detect a small but significant increase in protein carbonyls as a marker of oxidative stress (20).
Our discussion depends on measurements of biomarkers in serum while comments on oxidative stress in CNS. The reader should realize that this limitation is also the main reason of this study, that feasible serum markers (easy to perform and cheap) may avoid more complicated and costly procedures like MRI or CSF sampling. Actually, oxidants and antioxidants are generally permeable molecules, and disease states that cause a shift in their levels in any media will probably cause a comparable shift in their physiologic levels in serum. The major limitations of this study were the small number of patients and that all our patients were RRMS patients. The small number of patients also limited measurements of the influence of therapy duration or type of therapy on oxidative stress parameters. It could also be interesting to add another group of patients suffering a different neurological disease to compare the results of these parameters.
In conclusion, as we tried to describe, we strongly believe in the presence of oxidative stress in any stage of MS. We also believe in the superiority of measuring serum oxidative stress markers over sole measurements of iron load. We think TAS and TOS markers have failed to measure the oxidative stress in our patient group. However, there are contradictory results in other studies and the contradiction is a compulsion to further studies. Depending on our IMA results, we hypothesize that there is a dynamic production of ROS in MS patients which despite of a turnover, results in an accumulation in total amount of IMA in serum of these patients. To prove our hypothesis, serial follow-up serum IMA measurements at different stages of the disease are needed in order to inspect correlations of disease progress and levels of IMA. The efforts in developing clinical and imaging procedures are well appreciated, but a sensitive serum marker of oxidative stress is an urgent need in diagnosis and monitoring of these patients.


