Introduction
Breast cancer represents the most common malignancy in women. It is a complex disease with a highly variable clinical course and often a long-term development. In general, tumour development depends on the interaction between cancer cells and the surrounding non-malignant tissue composed of nonhematopoietic cells and immune cells. Cytokines, which probably have important effects on carcinogenesis, are mainly produced by host stromal and immune cells, in response to molecules produced by the cancer cells or as a part of inflammation that frequently accompanies tumour growth. Thus, they can be involved in the activation of immune effectors mechanisms that limit the growth of the tumour, but on the other hand, they can be involved in carcinogenesis, tumour growth, invasion and metastasis (1). In addition, malignant cells can produce cytokines in the same environment (1).
Results of the some recent studies confirm the presence of the interleukin 13 (IL-13 Th2 cytokine), in human cancers, including breast cancer (2,3). In some earlier investigations, it was found that NK cells (natural killer cells) isolated from blood and tumour tissue in patients with invasive ductal breast cancer produce interleukin 4 (IL-4) (4). Due to overlapping of the IL-4 and IL-13 immunoregulatory effects, it is possible that IL-13 is also present in blood and in the tumour tissue in patients with invasive breast cancer.
In some newer studies, it was reported that breast cancer tumours are infiltrated with CD4+ T cells secreting IFN-γ and IL-13. It is also found that CD4+ T cells promote the development of breast cancer tumours; actually breast cancer instructs dendritic cells to prime IL-13-secreting CD4+ T cells that facilitate tumour development (2). Furthermore, it was found that IL-13 and some other cytokines were over expressed in breast cancer by comparison with normal breast and it was also showed that IL-13 expression level was inversely correlated to estrogens receptor and progesterone receptor status (3), which implicates possible involvement of this cytokine in the aggressiveness of ER-negative breast tumours.
The aim of this study was to determine the presence and the expression of the IL-13 in primary breast cancer tumour tissue in relation to its expression in unchanged breast tissue of the same patients, and to the breast tissue of the patients with benign breast disease, and to investigate the correlation between the IL-13 expression levels and the pathohistological factors, and between IL-13 expression and the estrogens and progesterone receptor status.
Materials and methods
Patients
This study was approved by the Ethic committee of the University Clinical Centre Tuzla. It was prospective case-control study with well defined criteria for patient’s inclusion and exclusion.
The inclusion criteria were as follows:
- histologically proven invasive ductal breast cancer;
- no distance metastases;
- no previous adjuvant therapy;
- currently under no treatment;
- no other major illnesses.
All patients were subjected to the appropriate breast surgery at the Department of Surgery, University Clinical Centre Tuzla (all of them were females, Caucasians, residents of the narrow region in Tuzla surrounding, Bosnia and Herzegovina).
Tissue samples
Tissue samples have been obtained during surgery and were sent to the Department of pathology, Policlinic for Laboratory Diagnostics, University Clinical Centre of Tuzla, following the regular pathohistological examination and pathohistological diagnosis (PHD). According to the PHD findings, a total of 50 patients with the well documented invasive ductal breast cancer were included in this study: 24 with negative axillary’s lymph nodes, and 26 with positive axillary’s lymph nodes; and twenty patients with the finding of benign breast disease were included in the study also.
Tumour tissue samples and the samples of the surrounding unchanged tissue from patients with primary invasive ductal breast cancer were used, as well as breast tissue samples from patients with benign breast diseases.
Tumour tissue samples have been collected during twelve months, in the year 2008, and the final number of patients included in this investigation was determined according to the total number of patients with invasive breast cancer who have been subjected to the breast surgery in the University Clinical Centre during the year 2008, and who satisfy all inclusion criteria defined in this study. In the year 2008, 111 patients with invasive breast cancer have been subjected to the breast surgery, and from that number, patients without lymph node metastases for whom there was enough tissue material in paraffin blocs were included in this study. Number of patients with metastases was determined in relation to the number of patients without metastases and it was appropriate to it (proportional stratified sample).
Routine histological examination was performed with haematoxylin-eosin staining. All tumours were classified according to the criteria of the World Health Organization (5). Histological grade was obtained in accordance with a modified Scarff-Bloom-Richardson histological grading system. Staging was based on TNM (tumour-node-metastasis) system. Tumour size was evaluated separately (0.5-1.0 cm, £ 2 cm, 2-5 cm, > 5 cm).
Immunohistochemistry
Immunohistochemical staining for estrogens receptor (ER), progesterone receptor (PR) and interleukin 13 (IL-13) was performed on 4 mm thick formalin-fixed paraffin embedded sections. Deparaffinization and rehydration were performed in xylene and ethanol solutions (reducing concentration 96-70%). Sections were incubated in H2O2 solution (1.5% H2O2 in methanol) for 15 minutes to block endogenous peroxydase. Antigen retrieval were performed in procedure with the retrieval buffer (pH = 9.0, TRIS 20 mmol/L, EDTA 0.05 mmol/L, 0.05 % Tween 20) in a microwave oven by heating the slides for 15 minutes. After rinsing with the PBS buffer (100 mmol/L NaCl and 6 mmol/L Na2HPO4 x 2H2O), normal goat serum (for ER- and PR-staining) (DAKO, Golstrup, Denmark), and normal rabbit serum (for IL-13 staining) (Novocastra Newcastle Upon Tyne, UK), respectively, were applied for 15 minutes at room temperature to block non-specific antibody binding. Subsequently, sections were incubated with the primary antibody at 37 °C for one hour. After rinsing with the PBS buffer, the secondary antibody was applied for 30 minutes at 37 °C. A three-step technique was used for visualization with diaminobenzidine (Fluka Chemie GmbH, Buchs, Switzerland) as a chromogen. Counterstaining was performed with hematoxylin (Fluka Chemie, GmbH, Buchs, Switzerland). Slides were preserved in Canada balsam (turpentine).
Primary antibodies dilution used in this study were:
- A mouse antihuman monoclonal antibody against ER, clone NCL-ER-6F11 (Newcastle Upon Tyne, UK) 1:50 diluted in the PBS/BSA buffer pH = 7.2.
- A mouse antihuman monoclonal antibody against PR, clone NCL-PGR-312 (Novocastra Newcastle Upon Tyne,) 1:150 diluted in the PBS/BSA buffer pH = 7.2.
- A goat polyclonal antihuman IL-13, C-19 (Santa Cruz Biotechnology Inc., CA, USA) 1:50 diluted in the PBS/BSA buffer pH = 7.2.
Secondary antibodies dilutions used were:
- A goat antimouse polyclonal antibody biotin conjugated (DAKO, Golstrup, Denmark) 1:200 diluted in the PBS/BSA buffer pH = 7.2.
- A rabbit antigoat polyclonal antibody biotin conjugated (Santa Cruz Biotechnology Inc., CA, USA) 1:400 diluted in the PBS/BSA buffer pH = 7.2.
Tissue sections of breast cancer previously fortified index immunoreactivity were used as positive controls for estrogen and progesterone receptors and section of human heart tissue were used as positive control for IL-13. As negative controls were used sections from the same paraffin blocks as for positive controls but during immunohistochemical staining replacing a specific primary antibody with normal serum (the same species as primary antibody).
Immunohistochemical staining evaluation
The evaluation of the immunohistochemical staining, ER and PR was performed by a pathologist through a light microscopic observation (Olympus BX-50 light microscope, Olympus Medical Systems Corp. Tokyo, Japan). Evaluation was performed by using Remmeles immunoreactivity score (6) for ER- and PR- immunoreactivity analyzing. The extent of ER or PR immunoreactivity was scored as 0 for absence of the positive cells, +1 for less than 10% of positive cells, +2 for 11% to 50% positive cells, +3 for 51% to 80% of positive cells and +4 for more than 80% of positive cells. The intensity of ER or PR immunoreactivity was scored as 0 for no staining, 1 for weak staining, 2 for moderate staining and 3 for strong staining. The final score of the staining for each case was obtained by multiplying the extent of the immunoreactivity score with the intensity score. Cases with final score 0 were considered negative, with final score 1 to 3 considered weakly positive, with final score 4 to 8 considered moderately positive and with final score 9 to 12 considered strongly positive.
Due to some universally accepted criteria, the IL-13 expression scoring was developed by authors as the arbitrary system. Microscopy was done by two observers who have analysed the preparations together and done the last judgment.
The extent of IL-13 expression was scored as 0 for absence of the positive cells, +1 for 1% to 33% of positive cells, +2 for 34% to 66% of positive cells, +3 for more than 66% of positive cells. The intensity of IL-13 expression was scored as 0 for no staining, 1 for weak staining, 2 for moderate staining and 3 for strong staining. The extent of IL-13 expression score and the IL-13 intensity score were observed separately.
Statistical analysis
The distribution of the variables was determined using distribution histograms with probability plots. The results were evaluated by Chi-square test and with two-tailed Spearman correlation test considering value P < 0.05 statistically significant. Statistical analyses were performed in SPSS software (Version 17.0 SPSS Inc., Chicago, IL, USA).
Results
Tumour tissue samples and the surrounding tissue samples from 50 patients with the primary invasive ductal breast cancer were included in this study. Lymph node metastases were found in 26 patients (52%). Breast tissue from 20 patients with benign breast disease were also included and analysed in the study. Tumour size and histological grade were determined in 50 primary invasive ductal breast cancer patients. In patients with lymph node-negative breast cancer tumour size was > 5 cm in two patients, 2-5 cm in 10 patients, ≤ 2 cm in nine patients and 0.5-1.0 cm in three patients. In patients with lymph node-positive breast cancer tumour size was > 5 cm in three patients, 2-5 cm in 19 patients, ≤ 2 cm in three patients and 0.5-1.0 cm in one patient. In patients with lymph node-negative breast cancer tumour was weakly differentiated (histological grade III) in nine patients, moderately differentiated (histological grade II) in 13 patients, well differentiated (histological grade I) in two patients. In patients with lymph node-positive breast cancer tumour was weakly differentiated (histological grade III) in 14 patients, moderately differentiated (histological grade II) in 12 patients and there where no patients with well differentiated (histological grade I) tumour.
Estrogen and progesterone receptor status was also determined in patients with ductal invasive breast cancer. In patients with lymph node-negative breast cancer, estrogen receptors were positive in 18 patients and negative in six patients, and progesterone receptors were positive in 16 patients and negative in eight patients. In patients with lymph node-positive breast cancer, estrogen receptors were positive in 20 patients and negative in six patients, and progesterone receptors were positive in 17 patients and negative in nine patients. No significant differences between ER-IRS and PR-IRS were noticed considering the lymph node status. It was detected that ER-IRS significantly correlate to PR-IRS regardless to the lymph node status (in patients with no metastasis P < 0.001, in patients with metastasis P = 0.001, respectively). Statistically significant inverse correlation was found between ER-IRS and PR-IRS versus histological grade in lymph node-negative patients (r = -0.482, P = 0.017; r = -0.603, P = 0.002, respectively).
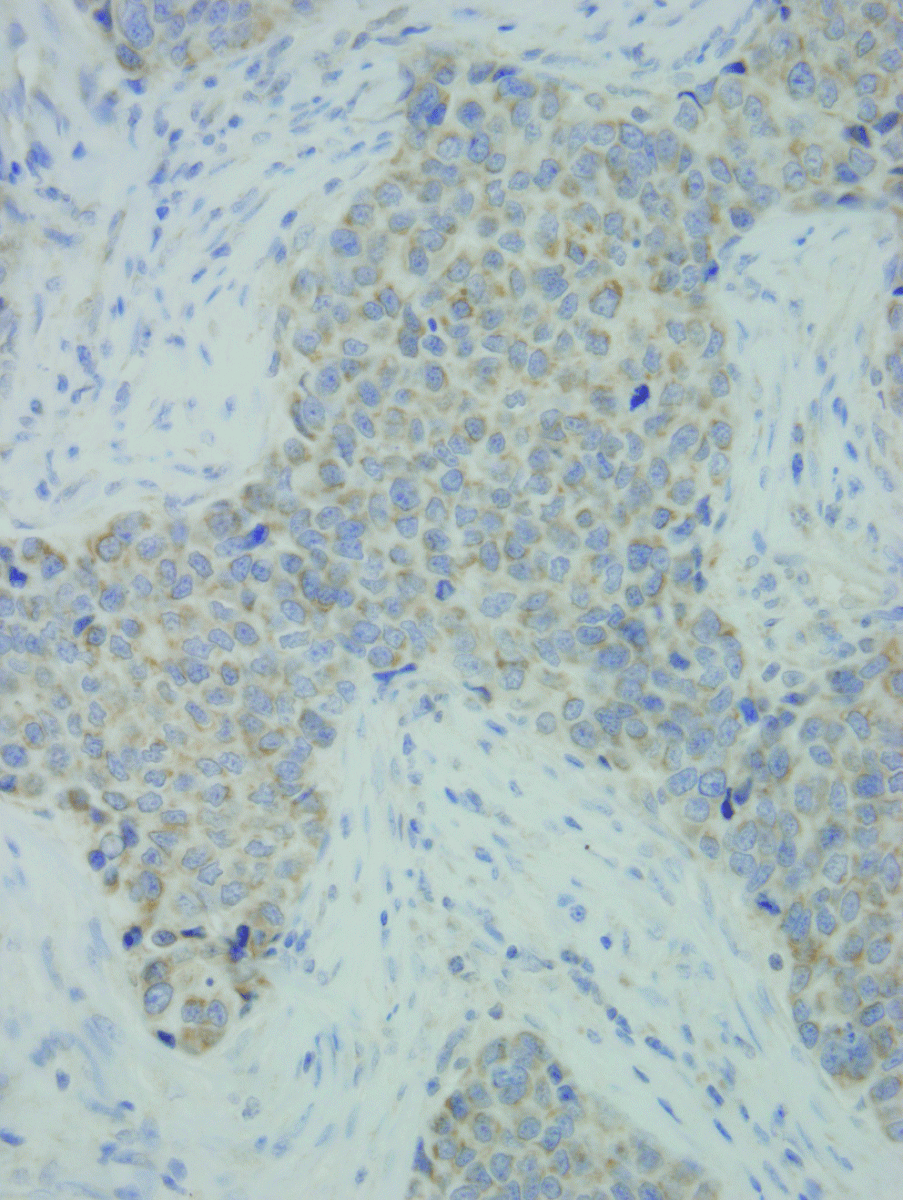
Figure 1. Microscopic appearance of breast cancer tumour tissue with the strong expression of IL-13 showed as immunohistochemical staining (brown staining) (HE; x40).
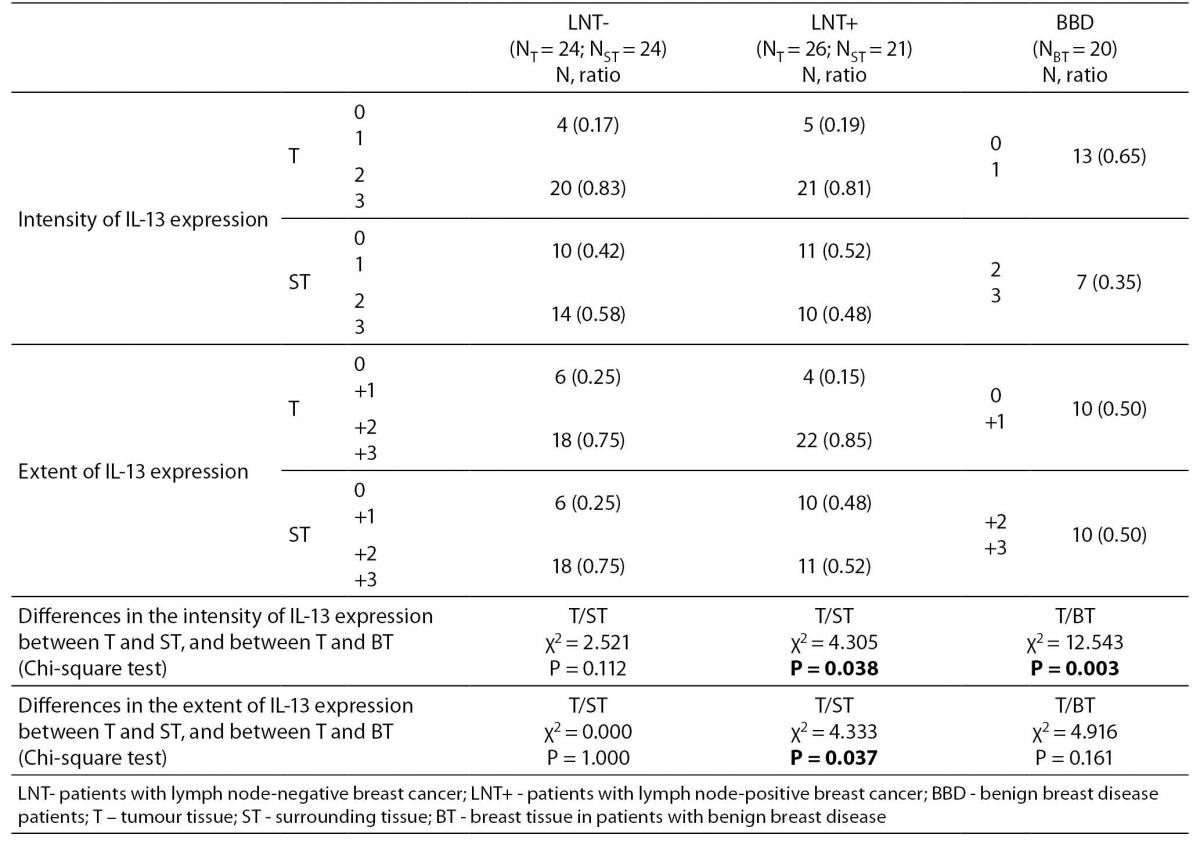
IL-13 was present in breast cancer tumour (Figure 1), in surrounding tissue of the same patients, and in breast tissue in patients with benign breast disease. Both the extent and the intensity of the expression of IL-13 were significantly higher in breast cancer tumour tissue compared to the surrounding unchanged tissue in the lymph node-positive patients, but not significantly higher in the lymph node-negative patients (Table 1). The IL-13 expression was significantly higher in breast cancer tumour compared with breast tissue of patients with benign breast diseases (Table 1).
Table 1. The intensity and the extent of IL-13 expression in patients without lymph node metastasis, with lymph node metastasis and in patients with benign breast disease
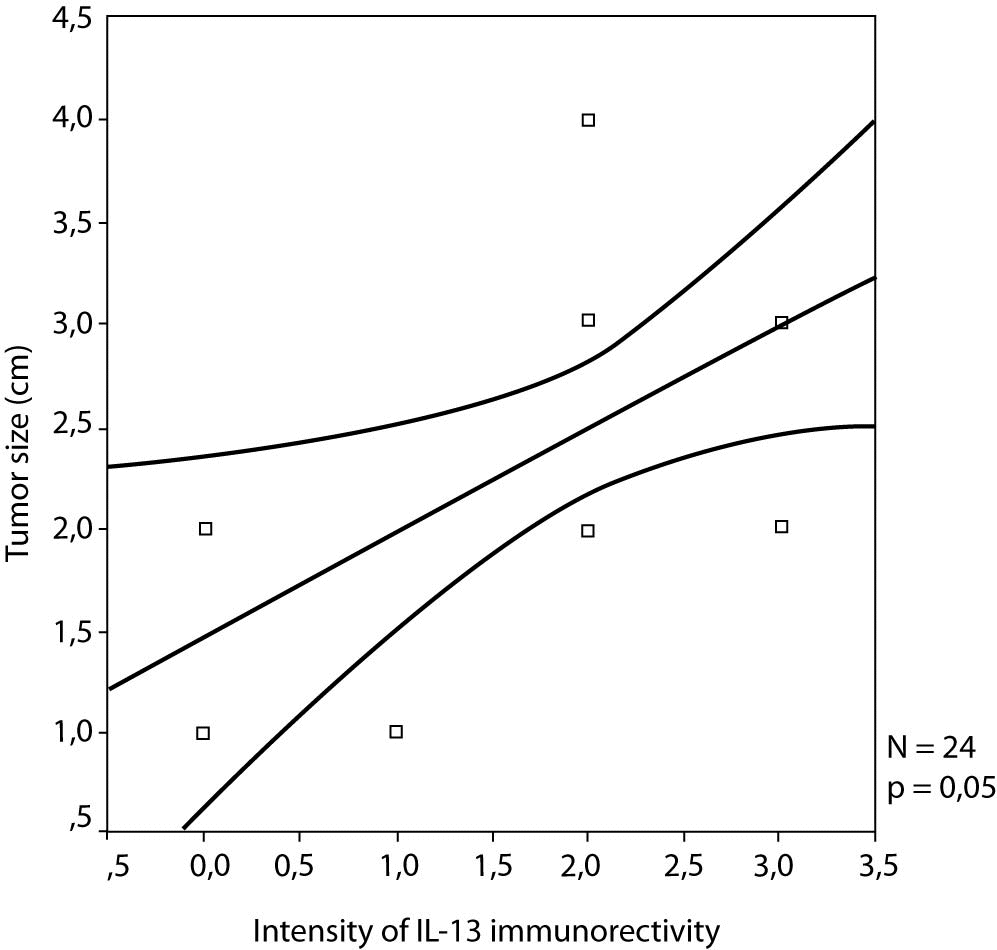
No statistically significant correlation between IL-13 expression and the lymph node status were noticed, as well as between IL-13 and pathohistological parameters, excluding statistically significant correlation between IL-13 expression and tumour size in patients with lymph node-negative breast cancer (r = 0.405, P = 0.050) (Figure 2).
Figure 2. The correlation between the intensity of IL-13 expression and the tumour size in lymph node-negative patients (r = 0.405; P = 0.050).
Discussion
The IL-13 expression was significantly higher in breast cancer tumour tissue compared with unchanged breast tissue in the same, lymph node-positive patients. These results are comparable with the results obtained in several other studies (2,3,7,8). Breast cancer cells also express pSTAT6 (2), while phosphorylation of STAT 6 is considered a signature of IL-13 signalling (and/or IL-4) (7), suggesting that IL-13 actually delivers signals to cancer cells (2). Furthermore, testing the mechanism by which procathepsin D influences the proliferation of cancer cells, it was found that breast cancer cells secrete IL-4, IL-8, IL-10, IL-13 and macrophage inflammatory protein-1β under procathepsin D induction (8). In other study it was shown that the human breast cancer tumours are infiltrated with dendritic cells, including immature myeloid dendritic cells in tumour beds and mature dendritic cells in peritumoral region (9). Mature dendritic cells were often found in clusters with CD4 T cells, suggesting an ongoing immune response, while dendritic cells infiltrating breast cancer tumours in humanized mice appeared responsible for the induction of IL-13-secreting CD4 T cells, at least in the initial phase of tumour development (2,9). There is an evidence that some cytokines are produced by host stromal cells and immune cells, in response to molecules secreted by the cancer cells or by the cancer cells, themselves (1,10). Therefore, neoplastic cells produce an array of cytokines and chemokines that are mitogenic and/or chemoattractants for granulocytes, mast cells, monocytes/macrophages, fibroblasts and endothelial cells. In addition, activated fibroblasts and infiltrating inflammatory cells secrete proteolytic enzymes, cytokines and chemokines, which are mitogenic for neoplastic cells, as well as endothelial cells involved in neoangiogenesis and lymphangiogenesis (10). These factors potentiate tumor growth, stimulate angiogenesis, induce fibroblast migration and maturation, and enable metastatic spread via engagement with either the venous or lymphatic networks (10). In accordance with all these literature data, it is possible that IL-13 investigated in this study, which was highly expressed in breast cancer tumour by comparison with unchanged breast tissue in the same patients, was also secreted by breast cancer cells. On the other hand, noticed presence of IL-13 in unchanged surrounding tissue and in breast tissue in the same time in patients with benign breast disease suggests that this cytokine has been probably produced both in response to surgical stress, wherefore patients involved in this study were exposed to (11,12), and/or as a part of inflammation process that often accompanies tumour growth (1).
Furthermore, results of some other studies have showed that cytokines IL-1β, IL-5, TNF-α, IL-6 and IL-2 were significantly associated with breast cancer disease and based on their serum levels, concentrations were higher in patients than in controls (13). However, no association was observed between investigated IL-13 gene polymorphism at positions -1512 A®C and -1055 C®T in the promoter and +2044 G®A in exon-4 and prognostic factors including tumour type, lymph node involvement and tumour size, and IL-13 serum level was undetectable in both patients and control subjects (14).
Results obtained in this study have showed that there were no differences in IL-13 expression between breast cancer patients with and without lymph node metastasis. Literature data about the involvement of IL-13 in breast cancer are poor. This result is unexpected and in some way contradictory and it is only possible to speculate about it. Cancer cells, in general, divide quickly and they have special metabolic requirements. In many cases these cells have higher biological potential in relation to normal cells. Furthermore, cancer cells differ among themselves regarding to their ability to migrate, and this feature seems to be sometimes individual and variable. These considerations might be, at least partly, possible explanation for the obtained results in this study for no significant difference in IL-13 expression between lymph node negative and lymph-node positive patients.
In relation to pathohistological factors, no significant correlations in IL-13 expression were found, excluding significant correlation between IL-13 expression and tumour size in patients with lymph node-negative breast cancer. Significant correlation of IL-13 to tumour size, although there was no significant correlation between IL-13 expression and the other pathohistological factors, implicate possible function of IL-13 as a growth factor for breast cancer tumour, and this is in accordance with some literature data (15,16). It was found that IL-13 and its receptors were frequently expressed by Hodgkin and Reed-Stenberg cells of Hodgkin lymphoma and may function as a growth factor for this tumour (15). Furthermore, it was reported that Th2 cytokines IL-4 and IL-13, expressed by tumour infiltrating lymphocytes isolated from patients with colon cancer cell, inhibited colon cancer cell-cell adhesion by down-regulation of E-cadherin, which may lead to tumour progression by dyscohesiveness of tumour cells (16).
Although no significant difference was observed in the IL-13 expression between the lymph node positive and the lymph node negative breast cancer patients, the over expression of IL-13 with regard to the surrounding tissue in these patients may, alone, indicate possible involvement of this cytokine in the complex pathogenesis of breast cancer but its mechanism is not yet enough clear. Some other recent data indicate that IL-13 receptor alpha 2 chain is over expressed in variety of human solid cancers, and that IL-13 receptor alpha 2 may serve as a prognostic biomarker of invasion and metastases in pancreatic cancer (17). Furthermore, IL-13 serum level determinations, and tumour cells morphologic analysis, when combined together may provide more detailed information about immunomodulating role of this cytokine in solid tumour tissue. That is important limitation to our study because it is not possible to precisely evaluate exact role of IL-13 in the immunomodulating process in the breast carcinogenesis, and measurements of IL-13 serum concentrations together with tumour cells histological analyses are needed. On the other hand, tissue expression estimation based on microscopy: immunochemical quantification of IL-13 is probably less objective in relation to some other approaches, for example the estimation of some house keeping gene product contents, and this is also possible limitation to this study. Nevertheless, the results obtained in this study may, at least partly, contribute to better understanding of the complex breast cancer aetiology, and may contribute to the development of the new diagnostic and therapeutic approaches to this severe disease.
Acknowledgments
We thank the staff of the Department of pathology, Policlinic for Laboratory Diagnostic, University Clinical Center Tuzla, for their technical assistance during the study. This work was supported by grant from Ministry of Education, Culture and Science of Canton Tuzla, Bosnia and Herzegovina, and we thank them.
Notes
Potential conflict of interest
None declared.
References
1. Smyth MJ, Cretney E, Kershaw MH, Hayakawa Y. Cytokines in cancer immunity and immunotherapy. Immunolo Rev 2004;202:275-93.
2. Aspord C, Pedroza-Gonzalez A, Gallegos M, Tindle S, Burton EC, Su D, et al. Breast cancer instructs dendritic cells to prime interleukin 13 – secreting CD4+ T cells that facilitate tumor development. J Exp Med 2007;204:1037-47.
3. Chawey C, Bibeau F, Gourgou-Bourgade S, Burlinchon S, Boissiere F, Laune D, et al. Oestrogen receptor negative breast cancers exhibit high cytokine content. Breast Cancer Res 2007;9:R15.
4. Lorenzen J, Lewis CE, McCraken D, Horak E, Greenal M. Human tumor associated NK-cells secrete increased amounts of interferon-γ and interleukin-4. Br J Cancer 1991;64:457-62.
5. Tavassoli FA, Devilee P. Tumours of the breast. In: Tavassoli FA, Devilee P, eds. World Health Organisation Classification of Tumours. Pathology and genetics of tumours of the breast and female genital organs. Lyon: IARC Press; 2003. p. 9-110.
6. Remmele W, Stegner HE. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 1987;3:138-40.
7. Skinnider BF, Elia AJ, Gascoyne RD, Patterson B, Trumper L, Kapp U, et al. Signal Transducer and activator of transcription 6 is frequently activated in Hodgkin and Reed-Sternberg cells of Hodgkin lymphoma. Blood 2002;99:618-26.
8. Fusek M, Vetvickova J, Vetvicka V. Secretion of cytokines in breast cancer cells: The molecular mechanism of procathepsin D proliferative effects. J Interferon Cytokine Res 2007;27:191-9.
9. Bell D, Chomarat P, Broyles D, Netto G, Harb GM, Lebecque S, et al. In breast carcinoma tissue, immature dendritic cells reside within the tumor, whereas mature dendritic cells are located in peritumoral areas. J Exp Med 1999;190:1417-26.
10. Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860-7.
11. Baigrie RJ, Lamont PM, Kwiatkowski D, Dallman MJ, Morris PJ. Systemic cytokine response after major surgery. Br J Surg 1992;79:757-60.
12. Yatsiv I, Morganti-Kossmann MC, Perez D, Dinarello SA, Novick D, Rubinstein M, et al. Elevated intracranial IL-18 in humans and mice after traumatic brain injury and evidence of experimental closed head injury. J Cereb Blood Flow Metab 2002;22:971-8.
13. Erdei E, Kang H, Meisner A, White K, Pickett G, Baca C, et al. Polymorphisms in cytokine genes and serum cytokine levels among New Mexican women with and without breast cancer. Cytokine 2010;51:18-24.
14. Faghih Z, Erfani N, Razmkhah M, Sameni S, Talei A, Ghaderi A. Interleukin 13 haplotypes and susceptibility of Iranian women to breast cancer. Mol Biol Rep 2009;36:1923-28.
15. Skinnider BF, Elia AJ, Gascoyne RD, Trumper LH, von Bonin F, Kapp U, et al. Interleukin 13 and interleukin 13 receptor are frequently expressed by Hodgkin and Reed-Sternberg cells of Hodgin Lymphoma. Blood 2001;97:250-5.
16. Kanai T, Watanabe M, Hayashi A, Nakazawa A, Yajima T, Okazawa A, et al. Regulatory effect of interleukin-4 and interleukin-13 on colon cancer cell adhesion. Br J Cancer 2003;82:1717-23.
17. Fujisawa T, Joshi B, Nakajima A, Puri RK. A Novel Role of interleukin-13 Receptor α2 in Pancreatic Cancer Invasion and Metastasis. Cancer Res 2009;69:8678-85.